significant figure of the refractive index being observed in different
parts of single large blocks of the most perfect glass. While such
minute and gradual variations are harmless for most optical purposes,
sudden variations which generally take the form of striae or veins
are fatal defects in all optical glass. In their coarsest forms such
striae are readily visible to the unaided eye, but finer ones escape
detection unless special means are taken for rendering them visible;
such special means conveniently take the form of an apparatus for
examining the glass in a beam of parallel light, when the striae
scatter the light and appear as either dark or bright lines according
to the position of the eye. Plate glass of the usual quality, which
appears to be perfectly homogeneous when looked at in the ordinary
way, is seen to be a mass of fine striae, when a considerable thickness
is examined in parallel light. Plate glass is, nevertheless, considerably
used for the cheaper forms of lenses, where the scattering of
the light and loss of definition arising from these fine striae is not
readily recognized.
Bubbles and enclosures of opaque matter, although more readily observed, do not constitute such serious defects; their presence in a lens, to a moderate extent, does not interfere with its performance (see above).
3. Hardness and Chemical Stability.—These properties contribute to the durability of lenses, and are specially desirable in the outer members of lens combinations which are likely to be subjected to frequent handling or are exposed to the weather. As a general rule, to which, however, there are important exceptions, both these qualities are found to a greater degree, the lower the refractive index of the glass. The chemical stability, i.e. the power of resisting the disintegrating effects of atmospheric moisture and carbonic acid, depends largely upon the quantity of alkalis contained in the glass and their proportion to the lead, lime or barium present, the stability being generally less the higher the proportion of alkali. A high silica-content tends towards both hardness and chemical stability, and this can be further increased by the addition of small proportions of boric acid; in larger quantities, however, the latter constituent produces the opposite effect.
4. Absence of Internal Strain.—Internal strain in glass arises from the unequal contraction of the outer and inner portions of masses of glass during cooling. Processes of annealing, or very gradual cooling, are intended to relieve these strains, but such processes are only completely effective when the cooling, particularly through those ranges of temperature where the glass is just losing the last traces of plasticity, is extremely gradual, a rate measured in hours per degree Centigrade being required. The existence of internal strains in glass can be readily recognized by examination in polarized light, any signs of double refraction indicating the existence of strain. If the glass is very badly annealed, the lenses made from it may fly to pieces during or after manufacture, but apart from such extreme cases the optical effects of internal strain are not readily observed except in large optical apparatus. Very perfectly annealed optical glass is now, however, readily obtainable.
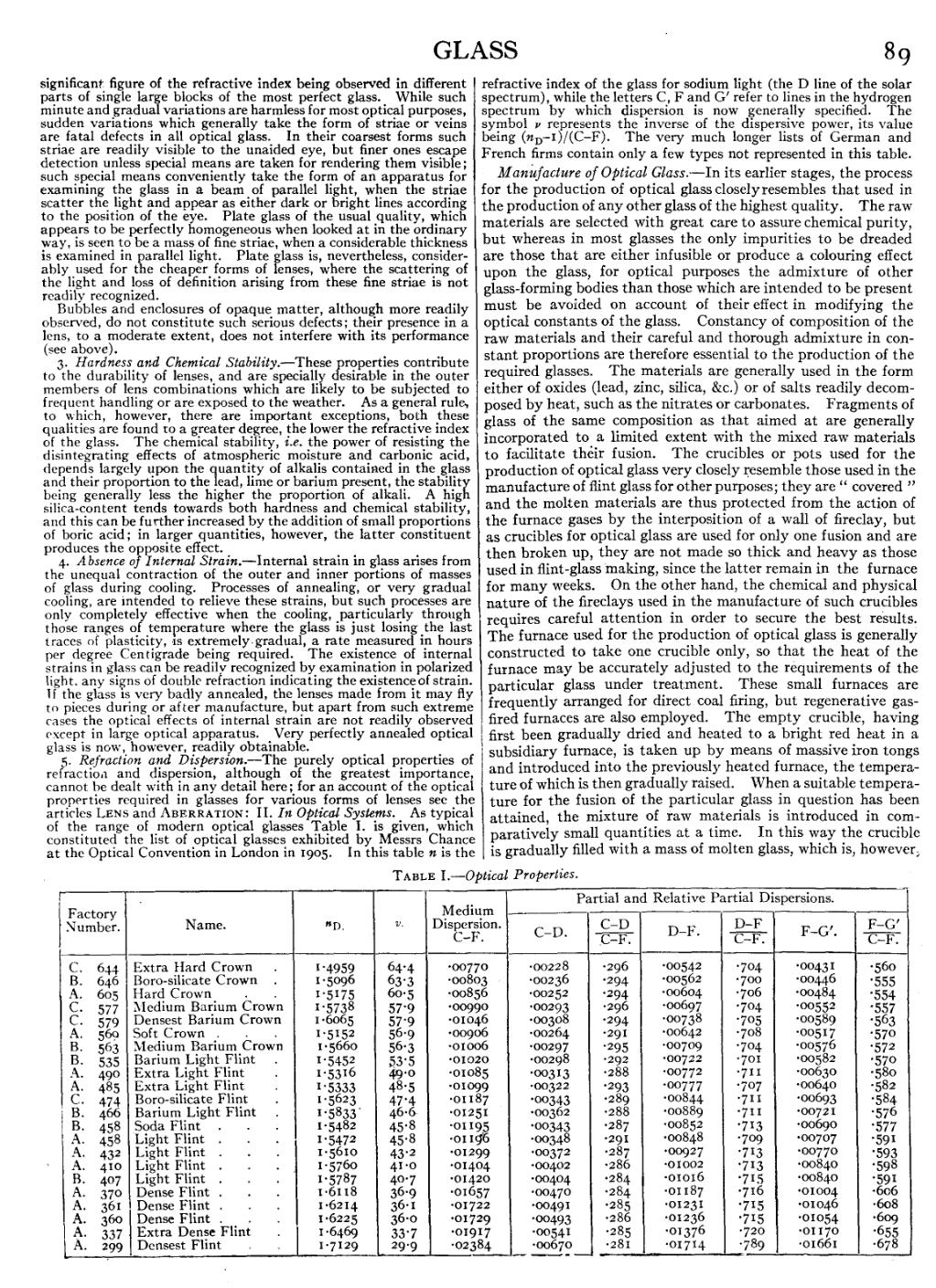
5. Refraction and Dispersion.—The purely optical properties of refraction and dispersion, although of the greatest importance, cannot be dealt with in any detail here; for an account of the optical properties required in glasses for various forms of lenses see the articles Lens and Aberration: II. In Optical Systems. As typical of the range of modern optical glasses Table I. is given, which constituted the list of optical glasses exhibited by Messrs Chance at the Optical Convention in London in 1905. In this table n is the refractive index of the glass for sodium light (the D line of the solar spectrum), while the letters C, F and G′ refer to lines in the hydrogen spectrum by which dispersion is now generally specified. The symbol ν represents the inverse of the dispersive power, its value being (nD−1)/(C−F). The very much longer lists of German and French firms contain only a few types not represented in this table.
| Factory Number. |
Name. | nD. | ν. | Medium Dispersion. C−F. |
Partial and Relative Partial Dispersions. | ||||||
| C−D. |
|
D−F. |
|
F−G′. |
| ||||||
| C. 644 B. 646 A. 605 C. 577 C. 579 A. 569 B. 563 B. 535 A. 490 A. 485 C. 474 B. 466 B. 458 A. 458 A. 432 A. 410 B. 407 A. 370 A. 361 A. 360 A. 337 A. 299 |
Extra Hard Crown Boro-silicate Crown Hard Crown Medium Barium Crown Densest Barium Crown Soft Crown Medium Barium Crown Barium Light Flint Extra Light Flint Extra Light Flint Boro-silicate Flint Barium Light Flint Soda Flint Light Flint Light Flint Light Flint Light Flint Dense Flint Dense Flint Dense Flint Extra Dense Flint Densest Flint |
1.4959 1.5096 1.5175 1.5738 1.6065 1.5152 1.5660 1.5452 1.5316 1.5333 1.5623 1.5833 1.5482 1.5472 1.5610 1.5760 1.5787 1.6118 1.6214 1.6225 1.6469 1.7129 |
64.4 63.3 60.5 57.9 57.9 56.9 56.3 53.5 49.0 48.5 47.4 46.6 45.8 45.8 43.2 41.0 40.7 36.9 36.1 36.0 33.7 29.9 |
.00770 .00803 .00856 .00990 .01046 .00906 .01006 .01020 .01085 .01099 .01187 .01251 .01195 .01196 .01299 .01404 .01420 .01657 .01722 .01729 .01917 .02384 |
.00228 .00236 .00252 .00293 .00308 .00264 .00297 .00298 .00313 .00322 .00343 .00362 .00343 .00348 .00372 .00402 .00404 .00470 .00491 .00493 .00541 .00670 |
.296 .294 .294 .296 .294 .291 .295 .292 .288 .293 .289 .288 .287 .291 .287 .286 .284 .284 .285 .286 .285 .281 |
.00542 .00562 .00604 .00697 .00738 .00642 .00709 .00722 .00772 .00777 .00844 .00889 .00852 .00848 .00927 .01002 .01016 .01187 .01231 .01236 .01376 .01714 |
.704 .700 .706 .704 .705 .708 .704 .701 .711 .707 .711 .711 .713 .709 .713 .713 .715 .716 .715 .715 .720 .789 |
.00431 .00446 .00484 .00552 .00589 .00517 .00576 .00582 .00630 .00640 .00693 .00721 .00690 .00707 .00770 .00840 .00840 .01004 .01046 .01054 .01170 .01661 |
.560 .555 .554 .557 .563 .570 .572 .570 .580 .582 .584 .576 .577 .591 .593 .598 .591 .606 .608 .609 .655 .678 | |
Manufacture of Optical Glass.—In its earlier stages, the process for the production of optical glass closely resembles that used in the production of any other glass of the highest quality. The raw materials are selected with great care to assure chemical purity, but whereas in most glasses the only impurities to be dreaded are those that are either infusible or produce a colouring effect upon the glass, for optical purposes the admixture of other glass-forming bodies than those which are intended to be present must be avoided on account of their effect in modifying the optical constants of the glass. Constancy of composition of the raw materials and their careful and thorough admixture in constant proportions are therefore essential to the production of the required glasses. The materials are generally used in the form either of oxides (lead, zinc, silica, &c.) or of salts readily decomposed by heat, such as the nitrates or carbonates. Fragments of glass of the same composition as that aimed at are generally incorporated to a limited extent with the mixed raw materials to facilitate their fusion. The crucibles or pots used for the production of optical glass very closely resemble those used in the manufacture of flint glass for other purposes; they are “covered” and the molten materials are thus protected from the action of the furnace gases by the interposition of a wall of fireclay, but as crucibles for optical glass are used for only one fusion and are then broken up, they are not made so thick and heavy as those used in flint-glass making, since the latter remain in the furnace for many weeks. On the other hand, the chemical and physical nature of the fireclays used in the manufacture of such crucibles requires careful attention in order to secure the best results. The furnace used for the production of optical glass is generally constructed to take one crucible only, so that the heat of the furnace may be accurately adjusted to the requirements of the particular glass under treatment. These small furnaces are frequently arranged for direct coal firing, but regenerative gas-fired furnaces are also employed. The empty crucible, having first been gradually dried and heated to a bright red heat in a subsidiary furnace, is taken up by means of massive iron tongs and introduced into the previously heated furnace, the temperature of which is then gradually raised. When a suitable temperature for the fusion of the particular glass in question has been attained, the mixture of raw materials is introduced in comparatively small quantities at a time. In this way the crucible is gradually filled with a mass of molten glass, which is, however,