Rays of Positive Electricity and Their Application to Chemical Analyses/Wien's Proof of the Magnetic and Electric Deflection of the Rays
W. Wien1 applied this method to demonstrate the magnetic and electric deflections of the positive rays; he proved in this way that the positive rays contained electrified particles, and the direction of the deflections showed that they were positively charged. He calculated by the formula we have just given the values of e\m and v for these particles.

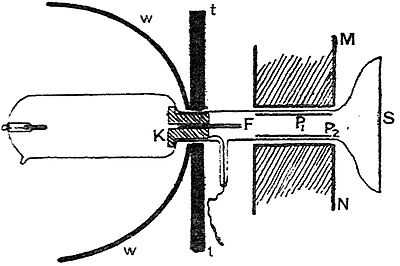
The method used by Wien is illustrated in Fig. 5.
The cathode K was an iron cylinder 3 cm. long with a hole 2 mm. in diameter bored throogh it, the anode was at the top of the tube. The lower end of the tube was made as flat as possible so as to facilitate the observation of the spot of luminosity produced by the impact of the positive rays on the glass. The magnetic fie!d was produced by an electromagnet poles were at N and S : it is necessary to shield the part of the tube through which the discharge is passing from the magnetic field; if this were not done the discharge would be so much affected by the magnet that trustworthy observations would be impossible; the tube was shielded by surrounding it with thick sheets of soft iron. The electrostatic field was produced between two parallel metal plates which were connected with the terminals of a voltaic battery. When the magnetic and electric fields were acting, the round spot of phosphorescence due to the positive rays coming through the hole In the cathode was drawn out into a straight band. Since the band was straight the velocities of the different particles producing it would all be the same; the values of e/m for these particles would, however, all be different. When the tube was filled with hydrogen, Wien found that the value of e/m for the most deflected portion was 7545, the value of e/m for a charged atom of hydrogen in the electrolysis of water is 10,000. In his first set of experiments Wien found that on filling the tube with oxygen the value of e\m for the most deflectible rays was 9800 in one experiment, in later experiments after very pure oxygen had flowed through the tube for a long time he found on first passing the discharge through the tube very much smaller values of e/m than for hydrogen, but the higher values reappeared after the discharge had passed for a short time.
The deflections of these rays by the electric and magnetic fields show that they are positively charged particles, the values of e\m obtained for these particles show also that they are much more massive than the particles in the cathode rays for which e/m =1.7 x 107. The displaced particles In this experiment were spread out into a continuous straight band, Indicating, according to the theory of the effect of electric and magnetic fields on charged particles, that in the positive rays there are particles giving all values of e\m from zero up to about 10,000. This would Imply, assuming that the charge on each particle is the same, that the masses of the particles vary continuously from a certain value comparable with the mass of an atom of hydrogen up to a value which Is very large in comparison with this mass. This continuous variation In the value of e/m is contrary to what might be expected, for, from the molecular theory of gases, the masses available In the gas would not vary continuously but would increase by finite steps, the smallest step being the mass of the atom of hydrogen: again the results of many different lines of investigation lead to the conclusion that e like m does not vary continuously, but that all electrical charges are multiples of a unit charge whose value in electrostatic measure Is 4.8 x 10-10. Again it would appear from the uniformity of the luminosity produced by the displaced positive rays that there is no special kind of atom which Is predominant among these rays. For if there had been a great excess of particles of one kind, these would have produced a very bright spot on the glass If they had all been moving with the same velocity, or a bright arc of a parabola if they had been moving with varying velocities. The experiments which I will now describe, which I made in 1906, show that the discrepancies between the theory and the experiments are due to the pressure of the gas In the discharge tube in Wien's experiments having been so high that the particles forming the positive rays collided with the molecules of the gas whilst they were passing through the electric and magnetic fields. The effect of these collisions is to ionize the gas so the gas through which the positive rays have to pass is full of charged particles, some charged with positive others with negative electricity. The result of the presence of this electrification is that some of the positive ray particles which were charged before they entered the electric and magnetic fields have their charges neutralized before they pass them, and thus do not experience the full deflection. On the other hand others which had got neutralized before they entered the field strike against a corpuscle or atom and get ionized by the collision, losing a negative corpuscle. In this way they acquire a positive charge In the field and are deflected by an amount which depends upon the stage in their journey at which they picked up the charge. Thus the quantities we denoted by A and B (see page 12) vary from particle to particle, and the values of e\m cannot be obtained from equations of the type (3) and (4) where A and B are cal culated on the supposition that the particles are charged for the whole of the time they are between the poles of the magnet and the plates of the condenser.

In my first experiments2 on this subject the arrangement was as follows: The cathode K (Fig. 6) had a hole bored through it and in this hole a tube F with a very fine bore was firmly fixed; it is essential to the success of the experiment that the bore of the tube should be exceedingly fine so as to get a small, well-defined patch when the positive rays strike the screen, S. This was a flat glass plate uniformly covered with powdered willemite which phosphoresces much more brightly than glass when struck by the rays. M and N are the poles of the electromagnet, and P1 P2 the parallel metal plates used to produce the magnetic and electric fields respectively; t,t, W,W are sheets of soft iron to screen the discharge taken from the magnetic field due to the electromagnet.
The effect observed on the screen depends to a very great extent upon the pressure of the gas in the tube; when this was not exceedingly low, the phosphorescence under the action of the magnetic and electric fields was drawn out into two continuous straight bands as in Fig. 7. The value of e/m for the most deflected portion of the band a, was 104, for that of band b, 5 X 103. These correspond to the values of e/m for the atom and molecule of hydrogen respectively, suggesting that the one band is due to hydrogen atoms, the other to hydrogen molecules. When the tube contains helium there are three bands to be seen as in Fig. 8. The values of elm at the tips of these bands are respectively 104, 5 x I03, 2.5 x I03, indicating that we have here again bands due to the atom and molecule of hydrogen, and in addition a new one due to atoms of helium, for (as the atomic weight of helium is 4) e\m for the helium atom is one quarter of that for the hydrogen atom. It is remarkable that the slope of these bands, and therefore, by page 12, the velocity of the particles, varies little if at all with the potential difference between the anode and cathode of the discharge tube. This potential difference may be increased three or four times without producing any appreciable effect upon the slope of the bands of phosphorescence. When air is in the tube, the appearances of the bands is much the same as when the tube contains hydrogen, though the phosphorescence is not so bright. The most conspicuous things on the screen in this case are the two bands corresponding to the atom and molecule of hydrogen respectively.
In addition to the two bands deflected in the direction indicating a positive charge on the particles, there is another fainter band deflected in the opposite direction which must therefore be due to particles with a negative charge. The value of elm for the tip of this band is 104, thus these negative particles are not cathode rays for which e/m is 1.7 x 107, but have a mass equal to that of an atom of hydrogen. The existence of particles deflected in the opposite direction to that of the majority of the particles had also been observed by Wien.
1 W. Wien, " Wied. Ann.," 65, p. 440, 1898; "Ann. der. Phys.," 8, p. 244,
2 J. J. Thomson, " Phil. Mag.," VI, XIII, p. 561.