1911 Encyclopædia Britannica/Cytology
CYTOLOGY (from κύτος, a hollow vessel, and λόγος, science), the scientific study of the “cells” or living units of protoplasm (q.v.), of which plants and animals are composed. All the higher, and the great majority of the lower, plants and animals are composed of a vast number of these vital units or “cells.” In the case of many microscopic forms, however, the entire organism, plant or animal, consists throughout life of a single cell. Familiar examples of these “unicellular” forms are Bacteria and Diatoms among the plants, and Foraminifera and Infusoria among the animals. In all cases, however, whether the cell-unit lives freely as a unicellular organism or forms an integral part of a multicellular individual, it exhibits in itself all the phenomena characteristic of living things. Each cell assimilates food material, whether this is obtained by its own activity, as in the majority of the protozoa, or is brought, as it were, to its own door by the blood stream, as in the higher Metazoa, and builds this food material into its own substance, a process accompanied by respiration and excretion and resulting in growth. Each cell exhibits in greater or less degree “irritability,” or the power of responding to stimuli; and finally each cell, at some time in its life, is capable of reproduction. It is evident therefore that in the multicellular forms all the complex manifestations of life are but the outcome of the co-ordinated activities of the constituent cells. The latter are indeed, as Virchow has termed them, “vital units.” It is therefore in these vital units that the explanation of vital phenomena must be sought (see Physiology). As Verworn[1] said, “It is to the cell that the study of every bodily function sooner or later drives us. In the muscle cell lies the problem of the heart beat and that of muscular contraction; in the gland cell reside the causes of secretion; in the epithelial cell, in the white blood corpuscle, lies the problem of the absorption of food, and the secrets of the mind are hidden in the ganglion cell.” So also the problems of development and inheritance have shown themselves to be cell problems, while the study of disease has produced a “cellular pathology.” The most important problems awaiting solution in biology are cell problems.
Historical.—The cell-theory ranks with the evolution theory in the far-reaching influence it has exerted on the growth of modern biology; and although almost entirely a product of the 19th century, the history of its development gives place, in point of interest, to that of no other general conception. The cell-theory—in a form, however, very different from that in which we now know it—was originally suggested by the study of plant structure; and the first steps to the formulation, many years later, of a definite cell-theory, were made as early as the later part of the 17th century by Robert Hooke, Marcello Malpighi and Nehemiah Grew. Hooke (1665) noted and described the vesicular nature of cork and similar vegetable substances, and designated the cavities by the term “cells.” A few years later Malpighi (1674) and Grew (1682), still of course working with the low power lenses alone available at that time, gave a more detailed description of the finer structure of plant tissue. They showed that it consisted in part of little cell-like cavities, provided with firm cell-walls and filled with fluid, and in part of long tube-like vessels. A long time passed before the next important step forward was made by C. L. Treviranus,[2] who, working on the growing parts of young plants, showed that the tubes and vessels of Malpighi and Grew arose from cells by the latter becoming elongated and attached end to end, the intervening walls breaking down; a conclusion afterwards confirmed by Hugo von Mohl (1830). It was not, however, until the appearance of Matthias Jakob Schleiden’s paper Beiträge zur Phytogenesis (1838) that we have a really comprehensive treatment of the cell, and the formulation of a definite cell-theory for plants. It is to the wealth of correlated observations and to the philosophic breadth of the conclusions in this paper that the subsequent rapid progress in cytology is undoubtedly to be attributed. Schleiden in this paper attempted to solve the problem of the mode of origin of cells. The nucleus (vide infra) of the cell had already been discovered by Robert Brown (1831), who, however, failed to realize its importance. Schleiden utilized Brown’s discovery, and although his theory of phytogenesis is based on erroneous observations, yet the great importance which he rightly attached to the nucleus as a cell-structure made it possible to extend the cell-theory to animal tissues also. We may indeed date the birth of animal cytology from Schleiden’s short but epoch-making paper. Comparisons between plant and animal tissues had already been made by several workers, among others by Johannes Müller (1835), and by F. G. J. Henle and J. E. Purkinje (1837). But the first real step to a comprehensive cell-theory to include animal tissues was made by Theodor Schwann. This author, stimulated by Schleiden’s work, published in 1830 a series of Mikroskopische Untersuchungen über die Übereinstimmung in der Structur und dem Wachstum der Tiere und Pflanzen. This epoch-making work ranks with that of Schleiden in its stimulating influence on biological research, and in spite of the greater technical difficulties in the way, raised animal cytology at one blow to the position already, and so laboriously, acquired by plant cytology. In the animal cell it is the nucleus and not the cell-wall that is most conspicuous, and it is largely to the importance which Schwann, following the example of Schleiden, attached to this structure as a cell constituent, that the success and far-reaching influence of his work is due. Another feature determining the success of Schwann’s work was his selection of embryonic tissue as material for investigation. He showed that in the embryo the cells all closely resemble one another, only becoming later converted into the tissue elements—nerve cells, muscle cells and so forth—as development proceeded; just as a similar mode of investigation had enabled Treviranus to trace the origin from typical cells of the vascular tissue in plants more than 30 years previously. And just as Treviranus showed that there was a union of cells to form the vessels in plants, so Schwann now showed that a union of cells frequently occurred in the formation of animal tissues.
So great was the stimulus given to cytological research by the work of Schleiden and Schwann that these authors are often referred to as the founders of the cell-theory. Their theory, however, differed very greatly from that of the present time. Not only did they suppose new cells to arise by a sort of “crystallization” from a formative “mother liquor” or “cytoblastema” (vide infra), but they both defined the cell as a “vesicle” provided with a firm cell-wall and with fluid contents. The cell-wall was regarded as the essential cell-structure, which by its own peculiar properties controlled the cell-processes. The work of Schleiden and Schwann marks the close of the first period in the history of the cell-theory—the period dominated by the cell-wall. The subsequent history is marked by the gradual recognition of the importance of the cell-contents. Schleiden had noticed in the plant cell a finely granular substance which he termed “plant slime” (Pflanzenschleim). In 1846 Hugo von Mohl applied to this substance the term “protoplasm”; a term already used by Purkinje six years previously for the formative substance of young animal embryos. Mohl showed that the young plant cell was at first completely filled by the protoplasm, and that only later, by the gradual accumulation of vacuoles in the interior, did this substance come to form a thin layer on the inner surface of the cell-wall. Mohl also described the spontaneous movement of the protoplasm, a phenomenon already noted by Schleiden for his plant slime, and originally discovered by Bonaventura Corti in 1772 for the cells of Chara, and rediscovered in 1807 by Treviranus. Not only was attention thus gradually directed to the importance of the cell-contents, but observations were not lacking, even in the plant kingdom, tending to weaken the importance hitherto attached to the cell-wall. Among these may be mentioned Cohn’s observation that in the reproduction of Algal forms the protoplasm contracts away from the cell-wall and escapes as a naked “swarm spore.” Similarly in the animal kingdom instances began to be noted in which no membrane appeared to be present (Kolliker, 1845; Bischoff, 1842), and for some time it was hotly debated whether these structures could be regarded as true cells. As a result of the resemblance between the streaming movements in these apparently naked cells (e.g. lymphocytes) and those seen in plant cells, R. Remak was led (1852–1853) to apply Mohl’s term “protoplasm” to the substance of these animal cells also. Similarly Max Schultze (1863) and H. A. de Bary (1859), as a result of the study of unicellular animals, came to the conclusion that the substance of these organisms, originally termed “Sarcode” by F. Dujardin, was identical with that of the plant and animal cell. Numerous workers now began to realize the subordinate position of the cell-wall (e.g. Nägeli, Alexander Braun, Leydig, Kolliker, Cohn, de Bary, &c.), but it is to Max Schultze above all that the credit is due for having laid the foundation of the modern conception of the cell—a conception often referred to as the proto-plasmic-theory in opposition to the cell-theory of Schleiden and Schwann. Max Schultze showed that one and the same substance, protoplasm, occurred in unicellular forms and in the higher plants and animals; that in plants this substance, though usually enclosed within a cell membrane, was sometimes naked (e.g. swarm spores), while in many animal tissues and in many of the unicellular forms the cell-membrane was always absent. He therefore concluded that in all cases the cell-membrane was unessential, and he redefined the “cell” of Schleiden and Schwann as “a small mass of protoplasm endowed with the attributes of life” (1861). In the same year the physiologist Brücke maintained that the complexity of vital phenomena necessitated the assumption for the cell-protoplasm itself of a complex structure, only invisible because of the limitations of our methods of observation. The cell in fact was to be regarded as being itself an “elementary organism.” By this time too it was realized that the formation of cells de novo, postulated by Schleiden’s theory of “phytogenesis,” did not occur. Cells only arose by the division of pre-existing cells,—as Virchow neatly expressed it in his since famous aphorism, omnis cellula e cellula. It was, however, many years before the details of this “cell-division” were laid bare (see Cell-Division below).
General Morphology of the Cell.—In its simplest form the cell is a more or less spherical mass of viscid, translucent and granular protoplasm. In addition to the living protoplasm there is present in the cell food-material in various stages of assimilation, which usually presents the appearance of fine granules or spherules suspended in the more or less alveolar or reticular mesh-work of the living protoplasm. In addition there may be more or less obvious accumulations of waste material, pigment, oil drops, &c.—products of the cell’s metabolic activity. All these relatively passive inclusions[3] are distinguished from the living protoplasm by the term “metaplasm” (Hanstein), or “paraplasm” (Kupffer), although in practice no very sharp distinction can be drawn between them. The cell is frequently, but by no means always, bounded by a cell-wall of greater or less thickness. In plants this cell-wall consists of cellulose, a substance closely allied to starch; in animals only very rarely is this the case. Usually the cell-wall, when this is present, is a product of the cell’s secretive activity; sometimes, however, it appears to be formed by an actual conversion of the surface layer of the protoplasm, and retains the power of growth by “intussusception” like the rest of the protoplasm. Even when a limiting membrane is present, however, evidence is steadily accumulating to show that the cell is not an isolated physiological unit, but that, in the vast majority of cases, there is a protoplasmic continuity between the cells of the organism. This continuity, which is effected by fine protoplasmic threads (“cell-bridges”) piercing the cell-wall and bridging the intercellular spaces when these are present, is to be regarded as the morphological expression of the physiological interdependence of the various—often widely separated—tissues of the body.[4] It is probable that it is the specialization of this primitive condition which has produced the cell-elements of the nervous system. In many cases the cell-connexions are so extensive as to obliterate cell-boundaries. A good example of such a “syncytial” tissue is provided by the heart muscle of Vertebrates and the intestinal musculature of Insects (Webber).[5]
In all multicellular, and in the great majority of unicellular, organisms the protoplasm of the cell-unit is differentiated into two very distinct regions,—a more or less central region, the nucleus, and a peripheral region (usually much more extensive), the cell-body or cytoplasm. This universal morphological differentiation of the cell-protoplasm is accompanied by corresponding chemical differences, and is the expression of a physiological division of labour of fundamental importance. In some of the simpler unicellular organisms, e.g. Tetramitus, the differentiated protoplasm is not segregated. Such forms are said to have a “distributed” nucleus, and among the Protozoa correspond to Haeckel’s “Protista.” It is probable that among plants the Bacteria and Cyanophyceae have a similar distributed nucleus. In all the higher forms, however, the segregation is well marked, and a “nuclear membrane” separates the substance of the nucleus, or “karyoplasm”[6] from the surrounding “cytoplasm.” Within the nuclear membrane the karyoplasm is differentiated into two very distinct portions, a clear fluid portion, the “karyolymph,” and a firmer portion in the form of a coarser or finer “nuclear reticulum.” This latter is again composed of two parts, the “linin reticulum,”[7] and, embedded in the latter and often irregularly aggregated at its nodal points, a granular substance, the “chromatin,”[8] the latter being the essential constituent of the nucleus. In addition to the chromatin there may be present in the nucleus one or more, usually spherical, and as yet somewhat enigmatical bodies, the “nucleoli.” In addition to the nucleus and cytoplasm, a third body, the “centrosome,” has often been considered as a constant cell-structure. It is a minute granule, usually lying in the cytoplasm not far from the nucleus, and plays an important part in cell-division and fertilization (see below).
Cell-differentiation.—Both among unicellular and multicellular individuals the cell assumes the most varied forms and performs the most diverse functions. In all cases, however, whether we examine the free-living shapeless and slowly creeping Amoeba, or the striped muscle cell or spermatozoon of the Metazoa (fig. 1, b and c), the constant recurrence of cytoplasm and nucleus show that we have to deal in each case with a cell. The variation in the form and structure of the cell is an expression of that universal economic law of nature, “division of labour,” with its almost invariable accompanying “morphological differentiation”; the earliest and most fundamental example being in the differentiation of the cell-protoplasm into cytoplasm and nucleus. In multicellular individuals the division of labour to which the structural complexity of the organism is due is between the individual cell-units, some cells developing one aspect, some another, of their vital attributes. Thus one cell specializes in, say, secretion, another in contractility, another in receiving and carrying stimuli, and so forth, so that we have the gland cell, the muscle cell, and the nerve cell, each appropriately grouped with its fellows to constitute the particular tissue or organ—gland, muscle or brain—which has for its function that of its constituent cells. In unicellular animals we also find division of labour and its accompanying morphological differentiation, but here there is no subdivision of the protoplasm of the organism into the semi-autonomous units which so greatly facilitate division of labour in the Metazoa; instead, division of labour must be between different regions of protoplasm in the single cell. The sharply defined character of this regional differentiation in the Protozoa, and the surprising structural complexity it may produce, sufficiently clearly show that although multicellular structure has greatly facilitated regional differentiation in the Metazoa, it is by no means essential to this process (see below, Present Position of the Cell-theory).
It is not within the scope of this article to attempt a comprehensive review of the variety in structural complexity to which this division of labour among the cells of the Metazoan and the regional differentiation of the cell-bodies of the Protozoa has given rise. Some indication of the wealth of variety may be best given by taking a general survey of cell-modifications, grouped according to the cell-attributes the expression of which they facilitate.

a and b from Schäfer’s Essentials of Histology, by permission of Longmans, Green & Co.
Fig. 1.—Types of Cells. a, Fat-cell enclosing a huge fat-globule. b, Part of a Mammalian “striated” muscle-cell (diagrammatic). c, Spermatozoa of mouse and bird.
(a) Structural Complexity facilitating Movement.—One of the most striking, and hence earliest described, of the fundamental attributes of protoplasm is its power of spontaneous movement. This is seen in the walled cell of plant tissue and in the naked cell-body of Amoeba. In the latter case the streaming movements of the naked protoplasm are accompanied by the formation of “pseudopodia,” and result in the highly characteristic “amoeboid” creeping movement of this and similar organisms (e.g. lymph corpuscles of the blood).[9] In these examples the whole protoplasm participates in the movement,—there has been no division of labour, and there is, therefore, no visible morphological differentiation. In many cells, movement (either of the entire body or of the surrounding medium) is by means of slender whip-like processes of the protoplasm flagella or cilia. These represent modified pseudopodia, and in the formation of the motile gametes of some of the lower forms, e.g. Myxomycetes (de Bary, 1859), Rhizopods (R. Hertwig, 1874), &c., the actual conversion of a pseudopodium into a flagellum can be witnessed. These vibratile processes may be either one or few in number, and are then large in size and move independently of one another; or they may be very numerous, covering the free surface of the cell (fig. 2, a); they are then very small and move strictly in unison. In the former case they are termed “flagella,” in the latter “cilia.” In some cases the flagellum is accompanied by an undulating membrane (e.g. Trypanosoma among the protozoa and in many spermatozoa), and it may be situated either at the front end (Euglena) or hind end (spermatozoa) of the body during motion. The cilia may form a uniform coating to the free surface of the cell, as in ciliated epithelium (fig. 2, a) and many infusoria, or the cilia may be variously modified and restricted to special regions of the body, e.g. the “undulating membrane” of the peristomial region in many infusoria, the swimming combs of the Ctenophora (q.v.), and the flame cells of the Platyelmia (q.v.). In one group of infusoria (Hypotricha), the cilia, “cirri,” have attained a high degree of differentiation, and reach a considerable size. Both cilia and flagella spring directly from the cell-protoplasm, piercing the cell-membrane, when this is present. At the point where they become continuous with the cell-body there is usually a deeply staining “basal granule.” In some cases the flagella are in direct connexion with the centrosome (see below, Cell-division), e.g. Trypanosoma and spermatozoa, in some cases even while the centrosome is functioning in mitosis (e.g. insect spermatogenesis, Henneguy[10] and Meves[11] (fig. 3).

From A. Gurwitsch, Morphologie und Biologie der Zelle, by permission of Gustav Fischer.
Fig. 2.—Types of Cells. a, Ciliated epithelial cells. (After Heidenhain.) b, Mucus-secreting “goblet”-cells. (After Gurwitsch.)

From O. Hertwig, Allgemeine Biologie, by permission of Gustav Fischer.
Fig. 3.—Spermatocytes of Bombyx mori, showing the precocious appearance of the spermatozoon flagellum and its relation to the centrosome. (After Henneguy.)
In the ability of Amoeba to contract into a spherical mass, and in the presence in its protoplasm of the contractile vacuole, we see another type of spontaneous movement—contractility—of the protoplasm. In the “musculo-epithelial” cells of Hydra, the elongated basal portion of the cell alone possesses this contractility. In the higher Metazoa the whole cell—muscle cell—is specialized for contractility, and shows, as a result of its specialization, a distinct fibrillation. This fibrillation is foreshadowed in the contractile regions of many Protozoa, e.g. in the cirri of hypotrichous Infusoria, the tentacle of Noctiluca, and the myophane layer of Gregarines. In the quickly contracting muscle cell of Vertebrates and insects, further specialization has produced a structure of considerable complexity (fig. 1, b). Here also the cell is fibrillated, but the fibrillae (sarcostyles) are much more distinct, and are segmented in a manner which gives to the entire cell a “cross striated” appearance. Since quick movement is usually (but not always) associated with voluntary control, these striated muscle cells are often termed “voluntary” muscle fibres. The great increase in length of these cells is accompanied by the fragmentation of the originally single nucleus.
(b) Cell-modification in Relation to Secretion.—Just as the complex movements considered above were the result of a great development of the power of spontaneous movement possessed by all protoplasm, so cell-secretion is the result of a development of the metabolic processes underlying all vital phenomena. But whereas specialization of the protoplasm for movement resulted in a very obvious morphological complexity, specialization for secretion results in molecular complexity, and only rarely and indirectly results in morphological differentiation. Usually indeed the specialization is only rendered evident by the appearance of the formed secretion, e.g. mucus-secreting epithelial cells (fig. 2, b), the ovarian ovum and the fat cell (fig. 1, a). In some cases a distinct fibrillation of the cytoplasm accompanies or precedes the appearance of the cell-secretion (Mathews, pancreas cell of Amphibia). In many cases the internal secretion is no mere accumulation, e.g. the internal skeleton of the Radiolaria, and the nematocysts of the Coelentera. Frequently in animal tissues the cell-secretions are accumulated in the intercellular spaces, and result in the formation of the various “connective tissues,” all of which are characterized by the immense amount of intercellular substance, e.g. fibrous tissue, cartilage and bone. Cell-modifications facilitating the general metabolism, but not necessarily indicating specialized secretion, also occur, e.g. the “gullet” of many Protozoa, the suctorial tubules of the Acinetaria, and the “nutritive processes” of the ovarian ova in many Lepidoptera. Mention may be made here of the network or canal system of the cytoplasm, described for many cells by Golgi, Holgren and others. An enigmatical structure, the “yolk-nucleus” of many ova, has been frequently regarded as a structure of considerable metabolic importance, e.g. Bambeke (1898) for Pholcus.[12]

Fig. 4.—Types of Nuclei.
From Prof. E. B. Wilson’s The Cell in Development and Inheritance, by permission of the author and of the Macmillan Co., New York.
a, Permanent spireme-nuclei in cells from the intestinal epithelium of a dipterous larva, Ptychoptera. (After van Gehuchten.)
From Korschelt and Heider, Lehrbuch der verg. Entwicklungsgeschichte der wirbellosen Tiere, by permission of Gustav Fischer.
b, Branched nucleus of the “nutritive” cell, from a portion of an ovarial tube of Forficula auricularia.
Striking modifications resulting from specialization in secretion are frequently presented by the nucleus. In many secreting cells this structure is extensively branched, e.g. many gland cells and ovarian nutritive cells of insects (fig. 4, b). In some cases the nucleus of the gland cell contains a persistent spireme thread (fig. 4, a); while almost all actively secreting cells are characterized by the possession of large or numerous nucleoli.

From Schäfer’s Essentials of Histology, by permission of Longmans, Green & Co.
Fig. 5.—Nervous and Sensory Cells.
A and B, Ganglion cells from the cerebral cortex; in A the only slightly branched axon may extend the whole length of the spinal cord. (After Schäfer.)
C, Body of a ganglion-cell showing “Nissl’s granules.”
D, Sensory cells from olfactory epithelium. (After Schultze.)
E, Diagrammatic representation of the sensory epithelium of retina (rod and cone layer). (After Schwalbe.)
(c) Specialization for the Reception and Conduction of Stimuli.—One of the most striking of the fundamental attributes of living protoplasm is its “irritability,” that is to say, its power of responding to external impressions, “stimuli,” by movement, which, both in kind and intensity, is wholly independent of the amount of energy expended by the stimulus. The stimulus conveyed by the nerve fibre to the muscle is out of all proportion to the amount of work it may cause the muscle to do. Although protoplasmic irritability is thus incapable of a simple mechanical explanation, science has rejected the assumption of a special “vital force,” and interprets protoplasmic response as being a long series of chemico-physical changes,[13] initiated, but only initiated, by the original stimulus; the latter thus standing in the same relation to the response it produces as the pull on the trigger to the propulsion of the rifle bullet. The function of receiving stimuli from the outer world, originally possessed to a greater or less extent by all cells, has, in the Metazoa, been relegated to one class of cells, the sensory cells[14] (fig. 5, D and E). Another class of cells—the “ganglion cells” or “neurones” (fig. 5, A and B), are concerned with the conduction of the stimuli so received. The contractile elements in the Metazoa are thus dependent for their stimuli on the nervous elements—the sensory cells and neurones.
Origin of Cells.—In the preceding sections we have considered the structure of the cell in relation to the fundamental attributes of cell-metabolism, irritability, and movement. We have now to consider the cell in relation to yet another vital attribute, that of reproduction. Just as we now know that the phenomena of assimilation, respiration, excretion, response, movement and so forth, characteristic of living things, are but the co-ordinated expressions of the corresponding activities of the constituent cells, so we now know that the reproduction of the organism is, in its ultimate analysis, a cell-process. Our knowledge of the essential fact that cells only arise by the division of pre-existing cells, now a fundamental axiom of biology, and of the details of this process, have been acquired during recent years by the strenuous efforts of numerous workers.[15] Matthias Jakob Schleiden (1838) supposed that in plants the new cell arose from the parent cell by a sort of “crystallizing” process from the cell fluid or “cytoblastema”; the nucleolus appearing first, then the nucleus, and finally the cell-body. Theodor Schwann (1839) extended Schleiden’s theory to animal tissues, with this yet greater error, that new cells might arise, not only within the mother cell as Schleiden had supposed, but also in the intercellular substance so common in animal tissues (to which he also gave the term “cytoblastema”). By 1846, however, the botanists, thanks mainly to the efforts of Hugo von Mohl and Nägeli, recognized as a general law that cells only arise by the division of a pre-existing cell. But it was long before the universal application of this law was recognized by zoologists; the delay being largely due to pathological phenomena. The work of Kölliker (1844–1845), Karl Bogislaus Reichert (1841–1847), and Remak (1852–1855), however, finally enabled Virchow in 1858 to maintain the law of the genetic continuity of cells in the since famous aphorism omnis cellula e cellula. At this time, however, nothing was known of the details of cell-division,—one school (Reichert, L. Auerbach, and the majority of the botanists) maintaining that the nucleus disappeared prior to cell-division, the other school (von Baer, Remak, Leydig, Haeckel, &c.) maintaining that it took a leading part in the process. It is not until the appearance of Anton Schneider’s work in 1873, followed by those of Fol, Auerbach, Strasburger and many others, that we begin to gain an insight into the process. In 1882 W. Flemming was able to extend Virchow’s aphorism to the nucleus also: omnis nucleus e nucleo.
Outline of Cell-division.—There are two very distinct methods of cell-division. The more general and also more complicated method is accompanied by the formation of a complex fibrillar mechanism, and was on this account termed “mitosis” (μίτος, a thread) by W. Flemming (1882), and “karyokinesis” (κάρυον, nut, nucleus, and κίνησις, change, movement) by W. Schleicher (1878). The other method, “amitosis,” or direct division, is unaccompanied by any visible mechanism and is of relatively exceptional occurrence. In the more usual method of cell-division, or “mitosis,” we can distinguish two distinct but parallel processes, the one undergone by the chromatin and resulting in the “chromatic figure,” the other usually only concerning the cytoplasm and resulting in the “achromatic figure.”[16]

a, b and c from Prof. E. B. Wilson’s The Cell in Development and Inheritance, by permission of the author and the Macmillan Co., New York; d from A. Gurwitsch, Morphologie u. Biologie der Zelle, by permission of Gustav Fischer
Fig. 6.—Diagram of Nuclear Division. a, Spireme stage; b, Spindle formed; c, Spindle complete; equatorial plate formed; d, Division completed.
We will consider the chromatin changes first. The chromatin granules lose their scattered arrangement on the nuclear reticulum, and become instead arranged in a linear series to form a coiled and deeply staining “spireme thread”[17] (fig. 6, a). As the thread contracts, its granular origin becomes less evident, and at the same time the coils become fewer in number; the “close” spireme of earlier stages becomes the “loose” spireme of later stages. As the spireme thread contracts, it segments into a number of short, and usually U-shaped, segments—the “chromosomes” (Waldeyer, 1888). The number of these chromosomes is always constant for the cells of any given species of plant or animal, but varies greatly in number in different species. Thus in the parasitic worm Ascaris megalocephala, var. univalens, there are only two. In the crustacean Artemia Bauer found 168, while in the amphibian Salamandra maculata, as also in the lily, the number is 24. While these changes have been proceeding in the nucleus, changes in the cytoplasm have resulted in the formation of the achromatic figure. These cytoplasmic changes are initiated by the division into two of a minute body, the “centrosome,” originally discovered by P. J. van Beneden in 1883,[18] and usually lying not far from the nucleus (fig. 6, a). The daughter centrosomes separate from one another, travelling to opposite poles of the nucleus. At the same time radiations extend out into the cytoplasm from the centrosomes, and, as the nuclear membrane disappears, invade the nuclear area (fig. 7, a). Some of the fibrillae in the latter region become attached to the chromosomes and are termed “mantle fibres”; others become continuous from one centrosome to the other and constitute the “spindle fibres.” The remaining radiations at the two poles of the spindle are the “astral rays.” (The details of the formation of the achromatic figure vary considerably, some indication of this is given in the next section in connexion with the question of the origin of the mitotic mechanism.) The chromosomes now arrange themselves in the “equatorial plate” of the spindle and each splits longitudinally into two[19] (fig. 6, b and c). The sister chromosomes now pass to opposite poles of the spindle (fig. 6, d), and there, returning to the “resting” condition, constitute the daughter nuclei. Division of the cell follows, usually, in animals, by simple constriction. Both Theodor Boveri and van Beneden, in their papers of 1887, regarded the centrosome as initiating, not only the division of the cell-body but that of the chromatin also; Beneden even suggested that the pull of the mantle fibres caused the division of the chromatin in the equatorial plate. W. Pfitzner in 1882 was the first to show that the splitting of the chromosomes in the equatorial plate was only the reappearance of a split in the spireme thread and was due to a corresponding division into two of each of the chromatin granules. In the spermatogenic cells of Ascaris, A. Brauer has shown that the chromatin granules divide while still scattered over the nuclear reticulum and before either the formation of a spireme thread or the division of the centrosome. In many other cases the reverse of this condition occurs, the centrosome dividing long before there is any indication of division in the nucleus (e.g. salamander spermatogenic cells, Meves, &c.). We must therefore, with Boveri and Brauer, regard the division of the chromatin in mitosis as a distinct reproductive act on the part of the chromatin granules, the chromosomes being merely aggregates (temporary or permanent, vide infra) of these self-propagating units.
For convenience of description it is usual to recognize four periods in mitosis: (i.) Prophase, (ii.) Metaphase, (iii.) Anaphase, and (iv.) Telophase (Strasburger, 1884). The prophase covers all changes up to the completion of the mitotic figure. The metaphase is the parting of the sister chromosomes in the equatorial plate; their passage to opposite poles of the spindle constitutes the anaphase; and their reconstruction to form the resting daughter nuclei, the telophase.
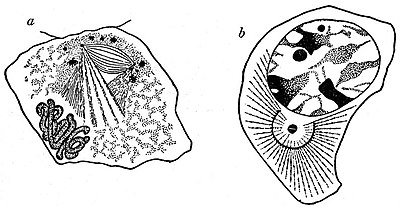
Fig. 7.—Centrosomes.
From Prof. E. B. Wilson’s The Cell in Development and Inheritance, by permission of the author and of The Macmillan Co., New York.
a, Leucocyte from a Salamander, showing permanent aster and centrosome.
From A. Gurwitsch, Morphologie u. Biologie der Zelle, by permission of Gustav Fischer.
b, Sperm-mother cell of Salamandra maculata, showing Hermann’s “central spindle.”
The Achromatic Figure.—The mode of origin of the achromatic figure varies greatly. In some cases a distinct and continuous spindle, the “central spindle” of F. Hermann, is visible from the very first separation of the daughter centrosomes (e.g. salamander spermatogenic cell)[20] (fig. 7, b). In other cases the rays only invade the nuclear area and become continuous in the equatorial plane after the centrosomes have assumed their definitive positions at the two poles of the nucleus, and may even appear to indent the disappearing nuclear membrane as they invade the nuclear area.[21] In the salamander testis cell (fig. 7, b), and in many other cases, the whole of the achromatic figure is obviously of cytoplasmic origin. In many cases, however, it equally obviously arises within the nucleus,[22] while in yet other cases[23] the spindle fibres are of mixed origin. The question, therefore, of the cytoplasmic or nuclear origin of the achromatic figure, at one time regarded as of considerable importance, is wholly immaterial. Various elaborate theories have been propounded to explain the mechanism of the mitotic figure. H. Fol (1873) regarded the centrosomes as centres of attractive forces, and compared the mitotic figure to the lines of force in the magnetic field, a comparison made by numerous subsequent workers. E. Klein’s hypotheses of two opposing systems of contractile fibrillae, elaborated by van Beneden (1883, 1887) and accepted by Boveri (1888), was still further extended by R. Heidenhain in relation to the leucocytes of the salamander, in which there is a permanent centrosome and astral rays to which the contractile movements of the cell appear to be due[24] (fig. 7, a). Hermann on the other hand confined the contractility to the astral and mantle fibres; while L. Druner regarded the spindle as exerting a pushing force, for not only do the interzonal spindle fibres elongate during the anaphase, but they were often at this period contorted, while on the other hand astral rays may be entirely absent (e.g. Infusoria), and in some cases the spindle pole may be caused to project at the surface of the cell. The futility of these attempted mechanical explanations of mitosis is sufficiently clearly shown, not only by the contradictory nature of the explanations themselves, but by the fact that, in amitosis, nuclear and cytoplasmic division occur without any fibrillar mechanism whatever.
Centrosome.[25]—This minute body was first detected at the spindle poles by Flemming in 1875, and independently by P. J. van Beneden in 1876. The important part played by the centrosome in fertilization,[26] first described by van Beneden and Theodor Boveri in their papers of 1887–1888, together with the behaviour of this structure in mitosis, led these authors to regard the centrosome not only as the dynamic centre of the cell but as a permanent cell-organ, which, like the nucleus, passed by division from one cell-generation to the next. This conclusion appeared to receive considerable support from the recognition of the centrosome in various kinds of resting cells,[27] and especially from the relation this structure frequently shows to the locomotor apparatus of the cell (e.g. its position in the centre of the radiating fibrillae in the contractile lymph and pigment cells, and its relation to the vibratile flagellum in spermatozoa and some protozoa, e.g. Trypanosoma).[28] In almost all cases the centrosome of the resting cell, when this can be detected, lies in the cytoplasm, and is often already divided in preparation for the next mitotic division (e.g. spermatogenic cells of the salamander; Meves). In some cases, however, it resides in, or arises from, the nucleus (Brauer; spermatogenesis of Ascaris, var. univalens). This indifferent nuclear or cytoplasmic position for the centrosome is paralleled by the attraction sphere or homologue of the centrosome in many Protozoa. Thus in many forms, e.g. Euglena (Keuten), it lies within the nucleus, while in other forms, e.g. Noctiluca (Ishikawa, 1894, 1898; Calkins, 1898) and Paramoeba (F. Schaudinn, 1896), it lies in the cytoplasm, while in Tetramitus it coexists with a “distributed” nucleus. In the Heliozoa conditions are exceptionally interesting; not only is the centrosome—here resembling in appearance that of the higher forms—permanently visible and extranuclear, lying at the centre of the radiations characteristic of these forms, but there is the strongest possible evidence for its formation de novo. For Schaudinn has shown in Acanthocystis that, in the formation of the swarm spores, the nucleus divides amitotically, the centrosome remaining visible and unchanged at the centre of the radiating processes. Yet a centrosome appears later in the nucleus of the swarm spores and migrates into the cytoplasm. The experiments of T. H. Morgan and E. B. Wilson, in which numerous centrosomes and asters (“cytasters”) are caused to appear in unfertilized sea-urchin eggs by a brief immersion in a 13% solution of magnesium chloride in sea-water,[29] as also the possibility in many cases that even in normal fertilization the cleavage centrosomes may arise de novo,[30] make it no longer possible to regard the centrosome as a permanent cell-structure.
Significance of Mitosis.—Whatever may be the nature of the chemico-physical changes occurring during cell-division, of which the achromatic spindle and astral rays are the visible expression, it is certain that the whole of this complicated process has for its function, not the division of the chromatin, for that has already occurred on the spireme thread or even earlier, but the distribution of the divided chromatin granules to the two daughter nuclei. It is indeed usually assumed that the mitotic mechanism is not merely for the distribution, but for the equal distribution, of the sister granules to the two daughter nuclei. The conspicuous part the chromatin is seen to play in the whole mechanism of heredity—in maturation, fertilization and development—indicating as it does that the chromatin is the chief, if not the only, bearer of the specific qualities of the organism, sufficiently clearly emphasizes the importance of the equal distribution of this substance between the daughter cells at successive cell-divisions. There are, however, serious objections to the interpretation of mitosis as an adaptation to ensure this equal distribution of the chromatin. Not only does the occurrence of amitosis show that the mitotic mechanism is not essential for either nuclear or cytoplasmic division, but direct division may occur[31] in the life-history of the germ cells, the very point at which it should not occur had mitosis the significance usually attached to it. On the other hand, the most elaborate mitosis occurs in cell-tissues (e.g. skin of salamander larva) which can take no possible share in the reproduction of the species. Moreover, we have no reason for supposing that the division of the chromatin in amitosis is not as meristic, and its subsequent distribution as equal, as is so visibly the case in mitosis.[32] It is necessary, therefore, to seek for some other explanation of the elaborate mechanism of mitosis than that which assumes it necessary for the equal distribution of the divided chromatin granules. The present writer believes the true explanation to be found in that great economic law of nature, “division of labour.” The same economy which, working under the control of natural selection, has produced the complexly differentiated tissues of the higher metazoa, which has led to the sexual differentiation between the conjugating gametes and thus to the sexual differentiation of the parents, has resulted in the production of mitosis. Only here the economy finds expression in division of labour, not in space, but in time. The work of the self-propagating chromatin granules is so ordered that periods of undisturbed metabolic activity alternate with periods of reproductive activity. The brief space of time occupied by the latter process has necessitated a more elaborate specialization of the forces—whatever their nature—controlling cell-division; a specialization which has resulted, just as a similar specialization in so many other cases has resulted, in a visible differentiation of the cell-protoplasm. This explanation is in harmony with the occurrence of typical mitosis in active tissue cells on the one hand, and of amitosis in the relatively quiescent primary germ cells on the other.
Individuality of the Chromosomes.—The most striking feature in the behaviour of the chromatin in mitosis is its resolution, at each division, into a—for any particular species—constant number of chromosomes. This constant recurrence of the specific number of chromosomes at every cell-division is capable of explanation in two radically different ways. One explanation assumes for the organism a specific peculiarity determining the segmentation of the spireme thread into a definite number of segments (Delage, 1899 and 1901).[33] The other regards chromosomes as independent units of the cell, retaining their identity between successive cell-divisions. The latter “Individualitäts Hypothese” was originally put forward by Theodor Boveri in 1887 as a result of C. Rabl’s observation (1885) that in epidermal cells of the salamander larva the chromosomes reappear in the mitosis of the daughter cells with the same arrangement as they possessed in the prophase of the mother cell—the angles of the U-shaped chromosomes being all directed towards one pole (Rabl’s “Poleseite”) of the nucleus. In the formation of the “resting” nucleus, the chromatin, becoming metabolically active, flows out on to the linin reticulum, all trace of the chromosomes being for the time lost. In Ascaris, Boveri (1888) obtained similar but still more striking results. The thickened ends of the four elongated chromosomes cause projections on the nuclear surface throughout the resting period, and the ends of the reappearing chromosomes always coincided with these protuberances; cf. also Sutton (1902) on locust spermatagonia. Moreover, the arrangement of the chromosomes must follow one of three well-marked groupings, and this is determined for each individual in the cleavage spindle of the egg and maintained throughout later development (fig. 8).

From Boveri’s Ergebnisse ü. d. Konstitution der chromatischen Substanz des Zellkerns, by permission of Gustav Fischer.
Fig. 8.—Preparation for Mitosis. a, Nucleus of “12 blastomere” of Ascaris megalocephala bivalens in resting condition; b and c, nuclei from sister 12 blastomeres in preparation for mitosis.
In the same worm (var. univalens) Boveri (1888 and 1899) found that occasional abnormalities in maturation resulted in the suppression of the first polar body and the inclusion of its chromosomes in the second maturation spindle; the egg-nucleus at the time of fertilization thus having two chromosomes instead of one, while the spermatozoon nucleus has only one. Three chromosomes instead of two reappear in subsequent divisions. Boveri’s “Individualitäts Hypothese” received striking support from the work of Herla (1893), L. R. Zoja (1895) and O. zur Strassen (1898). Herla and Zoja showed that if the egg of Ascaris megalocephala (var. bivalens), which possesses two chromosomes, be fertilized with the spermatozoon of var. univalens, in which the germ cell has only one chromosome and that smaller than either of the two in the other variety, three chromosomes reappear, two large and one small, in the cleavage divisions of the resulting hybrid embryo. Zur Strassen’s observations on the giant embryos of Ascaris also support Boveri’s theory. These embryos arise by the fusion of eggs, either before or after fertilization. The number of chromosomes in the subsequent cleavage-figures is proportional to the number of nuclei that have fused together. Similar results are given by Boveri’s (1893–1895) and T. H. Morgan’s (1895) experiments on the fertilization of enucleated sea-urchin egg-fragments; all the nuclei of the resulting embryo having only half the number of chromosomes characteristic of the species (e.g. in Echinus 9 instead of 18). All the above facts point to the conclusion that, as Boveri expressed it in his Grundgesetz der Zahlenkonstanz (1888), “the number of chromosomes arising from a resting nucleus is solely dependent on the number which originally entered into its composition.”[34]
Boveri’s Law of Proportional Nuclear Growth.—The chromatin in the nucleus is exactly halved at every cell-division. As the bulk of the chromatin remains constant from one cell-generation to another, it must double its bulk between successive divisions. That this proportional growth of the chromatin is dependent solely on the chromatin mass, and not on that of the cell, is very clearly indicated by cases where the normal chromatin mass has been artificially increased or reduced,[35] the chromatin in either case doubling its bulk between successive cell-divisions, and neither the mass of the chromatin nor the number of the chromosomes undergoing any readjustment. By double or partial fertilization, different regions in the same embryo may show nuclei of different sizes (Boveri). We must therefore distinguish in the cell between “young” and “adult” chromatin. In other words the chromatin must be regarded as being composed of individual units, each with a definite constant structure and maximum growth (Boveri, 1904). This conclusion is strongly suggested, not only by the evidence in favour of the individuality of the chromosomes considered above, but also by the independent reproductive activity of the chromatin granules in the prophase of mitosis.

From Boveri’s Ergebnisse ü. d. Konstitution der chromatischen Substanz des Zellkerns, by permission of Gustav Fischer.
Fig. 9.—Preparation for Mitosis. a, Spermatogonium of Brachystola magna with resting nucleus; b, Same with prophase for mitosis. (After Sutton.)
Differentiation among the Chromosomes.—If we grant the assumption of a persistent individuality for the chromosomes, then it becomes possible to consider whether in one and the same nucleus these structures may not take varying parts in controlling the cell’s activity in development and in inheritance. Such a differentiation among the chromosomes would be due to independent ancestry rather than to the economy resulting from a division of labour; nevertheless a division of labour of a sort would be the result of this gradual divergence of the chromosomes from one another, and we might therefore expect that, in some cases at least, a morphological would accompany the physiological differentiation. Examples of such a morphological differentiation do indeed occur in the “accessory” chromosomes first described by H. Henking (1891) for the spermatogonia of Pyrrhocoris, and since described for numerous other insects, Arachnids and Myriapods. W. Sutton’s work on the spermatogenesis of Brachystola magna is of especial interest in this connexion. Not only does the “accessory chromosome” in this insect form a resting nucleus independent, and obviously physiologically differentiated from that formed from the remaining chromosomes (fig. 9, a), but the latter are themselves differentiated by size, there being one pair of chromosomes of each size (fig. 9, b), a point of considerable interest when we remember that half the chromosomes in each cell are necessarily derived from each parent.[36]
Although this morphological differentiation among the chromosomes is undoubtedly to be regarded as indicating a corresponding physiological differentiation, it by no means follows that the latter need always, or even generally, be accompanied by the former. Since, however, the specific characters of the organism must be due to the combined activity of all the chromosomes, any physiological differentiation among the latter should result in abnormal development if the full complement of chromosomes be not present.[37] Boveri,[38] utilizing Herbst’s method[39] for separating echinoderm blastomeres, has interpreted in this manner the abnormal development which H. Driesch[40] found almost invariably to follow the double fertilization of the sea-urchin egg. In such eggs the first cleavage spindle is four-poled. The chromosomes are half again as numerous as in normally fertilized eggs (54 instead of 36), but each is only divided once, so that in the distribution of the resulting 108 chromosomes the four daughter nuclei receive each only 27 instead of 36 (assuming the distribution to be fairly equal, which is by no means usually the case in four-poled mitosis). Driesch had already (1900) shown that any one of the first four blastomeres of a normally fertilized egg will, if isolated, develop normally. Boveri found that in the case of the doubly fertilized egg the isolated “¼” blastomeres develop very variously, a variability only to be accounted for by their varying chromosome equipment. Occasionally a three-poled instead of a four-poled figure resulted from double fertilization. In such cases Driesch found, as we should expect from Boveri’s interpretation, that the percentage of approximately normal larvae was considerably greater; for not only would the chances of an equal distribution of the chromosomes be much greater, but the number received by each of the three daughter cells would approximate to, or even equal, the normal.
Reduction.—In all the Metazoa the prevailing, and in the higher forms the only, method of reproduction is by the union (conjugation) of two “sexually” differentiated germ-cells or “gametes”; a small motile “microgamete” or spermatozoon and a large yolk-laden “macrogamete” or ovum (see Reproduction). This differentiation between the germ-cells is another example of the advantages of division of labour; for while the onus of bringing about the union of the germ-cells is thrown entirely on the spermatozoon, the egg devotes itself to the accumulation of food-material (yolk) for the subsequent use of the developing embryo. Far more yolk is thus secreted than would be possible by the combined efforts of both the germ-cells had each of these at the same time to preserve its motility. The fundamental physiological difference which this division of labour has produced in the germ-cells is reflected on to the general metabolism of the parents and underlies the sexual differentiation of the latter.[41] Beyond this, however, sexual differentiation does not go. The two germ nuclei which enter into the formation of the first mitotic figure of the developing egg are not only physiologically equivalent, but, at the time of their union in the egg, are usually morphologically identical.[42] The essence of fertilization is, therefore, the union of two germ nuclei only differing from one another in that they are derived from separate individuals.[43] Since the number of chromosomes appearing in mitosis is solely dependent on the number which originally entered into the composition of the nucleus (Boveri’s Law of Chromosome-Constancy), it follows that, in the mitotic figures of the developing embryo, the chromosomes will be half maternal, half paternal in origin;[44] the germ nuclei thus necessarily possessing only half the number of chromosomes characteristic of the ordinary tissue cells of species, i.e. the somatic number.[45] The manner in which this “reduction” in the number of chromosomes in the germ-cells is brought about, and the significance to be attached to the process, constitute the most hotly debated questions in cytology. In all the metazoa the phenomenon of reduction is associated with the two last and, usually, rapidly succeeding “maturation” divisions by which the definitive germ-cells—ova or spermatozoa—are produced.[46]

From Korschelt and Heider’s Lehrbuch d. vergl. Entwicklungsgeschichte d. wirbellosen Tiere, by permission of Gustav Fischer.
Fig. 10.—Maturation Divisions. a–d, Formation of the tetrads in Cyclops. (After Rückert.) e, 1st maturation division; separation of the bivalent sister chromosomes. f, 2nd maturation division; distribution of the univalent chromosomes.

From Prof. E. B. Wilson’s The Cell in Development and Inheritance, by permission of the author and of the Macmillan Co., N. Y.
Fig. 11.—Maturation Divisions. Origin of the tetrads by ring formation in the spermatogenesis of the mole-cricket (Gryllotalpa) (vom Rath). a, Primary spermatocyte with six split, bivalent chromosomes. b and c, Split has opened out. d, Concentration of the chromatin has made visible the belated transverse division. e and f, Grouping of the completed tetrads in the equatorial plate of the first maturation division.
Assuming the persistent individuality of the chromosomes, then there are only three conceivable methods by which this numerical reduction can be brought about (Boveri, 1904, p. 60). (1) One-half the chromosomes degenerate. (2) The chromosomes are distributed entire, half to one daughter cell, half to the other (reducing division of Weismann, 1887). (3) The chromosomes fuse in pairs (Conjugation of the Chromosomes, Boveri, 1892). The first possibility—that of an actual degeneration of a part of the chromatin originally suggested by van Beneden and adopted by August Weismann, Boveri and others, has been long abandoned, and a steadily increasing bulk of evidence is tending to prove the general, if not universal, occurrence of the second method—the distribution between the daughter cells of undivided chromosomes. The occurrence of such a “reducing division” was postulated on theoretical grounds by Weismann (1887)[47] and by Boveri (1888); by the former as a result of his adoption of de Vries’s hypothesis of self-propagating and qualitatively varying units for the chromatin; by the latter in relation to his theory of chromosome individuality. The actual occurrence of this reducing division was first demonstrated by Henking (1891) for Pyrrhocoris, and afterwards by Häcker, vom Rath and many others, but especially by Rückert (1894) for Cyclops (fig. 10). In this latter type the chromatin of the oocyte, as this prepares for the first maturation division, resolves itself into 12 (instead of 24) longitudinally split chromosomes (fig. 10, a). As these continue to thicken and contract a transverse fission appears (fig. 10, c). This is to be regarded as a belated segmentation of the spireme thread, and shows that the reduction so far is only a “pseudo-reduction” (Rückert), the chromosomes being really all present but temporally united in pairs, i.e. “bivalent” (Häcker). A striking confirmation of this interpretation is provided by Korschelt’s description of reduction in the annelid Ophryotrocha. In this type the full somatic number of split chromosomes (here only four) appears, and these secondarily associate end to end in pairs, thus forming split “diads” (i.e. tetrads), in every way similar to those described by Rückert for Cyclops. In the latter type, at the first maturation division, the sister diads are separated from one another, an “equating” division thus taking place. At the second division the diads are resolved into their constituent parts, and the “univalent” chromosomes are distributed to the daughter cells (reducing division). A similar process has since been described for numerous other types (e.g. various arthropods, Häcker, 1895–1898; vom Rath, 1895; and by Sutton for Brachystola, 1902–1903). In Ophryotrocha, as in Pyrrhocoris (Henking), Anasa (Paulmeir), Peripatus (Montgomery), &c., reduction occurs at the first maturation division (“pre-reduction” of Korschelt and Heider, 1900), instead of at the second division (post-reduction) as in most Copepods and Orthoptera. In many cases the tetrads (i.e. split chromosomes associated in pairs) have the form of rings, the genesis of which was first clearly determined by vom Rath (1892) in the mole cricket Gryllotalpa (fig. 11). In this form the sister diads remain united by their ends but widely separate in the middle (fig. 11, b). As in Cyclops, the belated transverse segmentation appears as the condensation of the chromatin proceeds (fig. 11, d), but the symmetrical tetrads which this process here produces make it impossible to determine at which of the two divisions reduction is effected. An essentially similar ring formation occurs in Enchaeta and Calanus (vom Rath), and in the Copepods Heterocope and Diaptomus (Rückert), and in other types.[48]

From O. Hertwig, Allgemeine Biologie, by permission of Gustav Fischer.
Fig. 12.—Heterotypical Mitosis. (Schematic, after Flemming.)
All the above cases, in which the reduction is effected by the distribution of entire chromosomes at one or other of the maturation divisions, may be grouped together as “pseudomitotic” (Häcker, and Korschelt & Heider). In sharp contrast to the pseudomitotic method is the “Eumitotic” method, in which the chromosomes are longitudinally divided at both divisions. Such a method not only robs the process of any “reducing” value in Weismann’s sense, but is in serious conflict with the chromosome-individuality hypothesis. Nevertheless it is in this sense that Boveri (1881) and van Beneden (1883–1887) described the maturation of the egg, and at a later period Brauer (1893) that of the spermatozoon, in Ascaris. In each case the tetrads are formed by the double longitudinal splitting of the chromosomes, the latter appearing in the prophase in the reduced number. Not only was the eumitotic method of Ascaris the first method to be described, but the descriptions are fully equal in point of clearness to that of Hertwig for the pseudomitotic maturation of Cyclops.[49] A similar eumitotic maturation has been described for other types also, e.g. Sagitta and the Heteropods, but nowhere more frequently than in the Vertebrates among animals and the Phanerogams among plants. In these two latter groups the chromosomes of the reducing division only rarely have a ring form comparable to that seen in Gryllotalpa, &c. When such rings do occur their genesis is very obscure, and at no time do they present the appearance of “tetrads.” It is the characteristic appearance these looped chromosomes give to the first maturation division in many Vertebrates, and especially in the Amphibia (fig. 12), that originally led Flemming (1887) to term this type of mitosis “heterotypical”; the second division, lacking this peculiar appearance, being distinguished as “homotypical.” Until quite recently these looped chromosomes of the heterotypical mitosis of Vertebrates (and plants) were described as arising by the opening out of longitudinally split chromosomes, exactly as this occurs in the early prophase of the maturation divisions in such types as Gryllotalpa, Diaptomus, &c. In the heterotype mitosis, however, no transverse segmentation appears, and the halves of the rings, as they separate in the first division, show an obvious longitudinal split in preparation for the second division.[50] Both divisions were thus interpreted as equating divisions.[51] The more recent works of Farmer and Moore (1903–1905), Montgomery (1903, Amphibia), and (for plants) Strasburger (1903–1904) have shown, however, that even for the higher plants and animals, a reducing division in Weismann’s sense occurs in an essentially similar manner to that so convincingly described by Rückert, vom Rath and others, for Invertebrate types. For the chromosomes of the heterotype mitosis arise by the looping round, not opening out, of the bivalent chromosomes. The first division is thus a reducing division, while the split appearing in the anaphase of the heterotype and presumably reappearing in the prophase of the homotype is the original split of the spireme thread.
The widespread, if not universal, formation of tetrads, i.e. the temporary union in pairs of split chromosomes, in reduction, and the relation this latter process always bears to two rapidly succeeding maturation divisions—those completing the gametogenic cycle in animals and terminating the sporophytic generation in plants,—has received a suggestive explanation at the hands of Boveri (1904). The growth of the chromatin is an indispensable prelude to its reproduction (Boveri’s Law of Proportional Growth). The chromatin is therefore incapable of undergoing reproductive fission in two successive mitotic divisions when these are not separated by a resting (i.e. growth) period. In addition to this, the “bipolar” condition of the adult chromosomes, which determines its mode of attachment to mantle fibres from both poles of the spindle, is not possessed by the unripe chromatin. The undivided, i.e. unripe, chromosomes are therefore incapable of utilizing the mitotic mechanism for such a transverse fission as Weismann originally postulated. The difficulty is, however, at once overcome if the unripe chromosomes are associated in pairs in the equatorial plate, for the bivalent chromosomes so produced are bipolar just as are the adult (i.e. split) chromosomes in the ordinary and homotype mitosis.[52]
Synopsis (συνάπτειν, to fuse together).—During the prophase of the reducing or heterotype divisions the whole of the chromatin becomes temporarily massed together at one pole of the nucleus (Moore, 1896, for Elasmobranchs). Montgomery (1901) has suggested that this is to facilitate the temporary union in pairs, or “conjugation” of homologous paternal and maternal chromosomes. In Ascaris megalocephala var. univalens, where the somatic number is only two, the association must necessarily be between homologous chromosomes. The assumption that this “selective pairing” of equivalent chromosomes is universal is supported by the behaviour of the “Heterochromosomes” (Montgomery) of the Hemiptera. These chromosomes, distinguished by their size, are paired before, and single after, the “pseudo-reduction” has taken place. Even more convincing is Sutton’s account of reduction in Brachystola already referred to.[53] Boveri (1904) has suggested that this temporary association of the chromosomes—presumably facilitated by the synapsis—has a much deeper meaning than to ensure their correct distribution between the daughter nuclei in the heterotype mitosis; the associated chromosomes exchanging material in a manner analogous to conjugation in Paramoecium.[54]
Present Position of the Cell-theory.—Since the time of Schleiden and Schwann a wealth of evidence has accumulated in support of the “cell-theory”—the theory which regards the cell as the unit of organic structure. “The organism consists morphologically, of cells, and subsists, physiologically, by means of the ‘reciprocal action’ of the cells,”—this was the cell standpoint of Schleiden and Schwann, and it is no exaggeration to say that this same conception has dominated the cell-theory almost to the present day.[55] The frequently striking correlation between cell-division and cell-differentiation in development has caused this process to be regarded as dependent on cell-division, while a wholly exaggerated importance has been attached to the distinction between “unicellular” and “multicellular” organisms—between “intercellular” and “intracellular” organs. The influence of the “cells” upon one another, the subordination of the cell’s growth, division and differentiation, to the requirements of the whole organism—seen in normal growth, but nowhere more strikingly than in development and regeneration,—is, however, very difficult of explanation in terms of the cell-theory as this was, until quite recently, generally understood. The very elaborate regional differentiation of the protoplasm often seen in the Protozoa sufficiently indicate that multicellular structure is no essential condition for complex regional differentiation. That the regional differentiation of the protoplasm in the Metazoa should usually correspond with cell-limits is scarcely surprising. Nor is it to be wondered at that, with so convenient a mechanism for segregation to hand as cell-division, the progressive differentiation seen during development should often appear to go hand in hand with this process. In recent years, however, evidence has been steadily accumulating to show that this association between cell-division and regional differentiation of the protoplasm in development is a casual one—as casual, and as natural, as the correspondence between cell limits and regional differentiation in the formed tissues. The fact that the regional differentiation may be foreshadowed in the egg before cleavage begins,[56]—that as Driesch has shown, the mode of cleavage may be artificially altered without affecting the ultimate organization of the embryo,—and many other similar observations, tend to emphasize the importance of the “organism” standpoint (C. O. Whitman, 1903, p. 642) in contradistinction to the widely prevalent “cell” standpoint. The occurrence of syncytial organs and organisms, and the increasing frequency with which protoplasmic continuity is being demonstrated between all kinds of cells, are facts tending in the same direction. In the plant kingdom the growth of the mass has been recognized as the primary factor in development;[57] die Pflanze bildet Zellen, nicht die Zelle bildet Pflanzen (de Bary). For the animal kingdom this “Inadequacy of the Cell-Theory of Development” has been maintained amongst others by Whitman,[58] and by Adam Sedgwick.[59] The latter author, mainly as the result of work on the development of Peripatus and of Elasmobranch embryos, regards the developing embryo as a continuous protoplasmic reticulum, for the nuclei of which the limiting epithelial layers constitute as it were a breeding ground. Differentiation is a regional specialization of this nucleated meshwork, and is not to be regarded as the result of the proliferation and subsequent specialization of cells predestined by cleavage for this end.
It is possible to suggest a mechanico-physical explanation of multicellular structure which will deprive the cell of much of its assumed significance as a unit of organization. The fact that surface area becomes relatively less extensive as bulk increases would alone set a limit to the size of “unicellular” organisms; for not only is there a constant reaction between nucleus and cytoplasm through the nuclear membrane, but the surface of the cell serves both for the intake of food and the elimination of waste material. In addition to the limit thus imposed upon the cytoplasmic area which can be effectually controlled by the nucleus, and the necessity for a minimum surface area to the protoplasmic mass, the advantages of the more or less complete subdivision of the living substance into—as far as their metabolism is concerned—semi-autonomous units, is indicated by the mechanical support derived from the specialized cell walls and turgescent cells of the plant, and the intercellular secretions of the animal tissues. It is more than possible that these two conditions—i.e. surface area for diffusion, and mechanical support—are alone responsible for the origin of multicellular structure, and that the sharply defined character this now so generally possesses has been secondarily acquired as a result of the facilities it undoubtedly offers for regional specialization in the protoplasmic mass.
Bibliography.—The special literature of cytology has grown to large dimensions. The following are the more important text-books and papers of general interest: E. B. Wilson, The Cell in Development and Inheritance (2nd ed., 1900); A. Gurwitsch, Morphologie und Biologie der Zelle (Jena, 1904); O. Hertwig, Allgemeine Biologie (Jena, 1906); Korschelt and Heider, Lehrbuch der vergl. Entwicklungsgeschichte der wirbellosen Tiere, Allgem. Teil, “The Germ Cells and Experimental Embryology” (Jena, 1903); Whitman, “The Inadequacy of the Cell Theory of Development,” Journ. Morph. viii., 1893; Adam Sedgwick, “On the Inadequacy of the Cellular Theory of Development,” Quart. Journ. Micro. Science, xxxvii.; G. C. Bourne, “A Criticism of the Cell Theory” (an answer to Sedgwick’s paper), Quart. Journ. Micro. Science, xxxviii.; Th. Boveri, “Befruchtung,” Merkel-Bonnets Ergebnisse der Anat. u. Entwicklungsgesch. Bd. i. (1892), Das Problem der Befruchtung (Jena, 1902), Ergebnisse über die Konstitution der chromatischen Substanz des Zellkerns (Jena, 1904); J. Rückert, “Die Chromatinreduktion bei der Reifung der Sexualzellen,” Merkel-Bonnets Ergebnisse, Bd. iii. (1894); V. Häcker, “Die Reifungserscheinungen,” Ergebn. Anat. u. Entwicklungsgesch. Bd. viii. (1898); F. Meves, “Zellteilung,” Merkel-Bonnets Ergebnisse, Bd. viii. (1898, 1899); W. Waldeyer, “Die Geschlechtszellen,” in O. Hertwig’s Handbuch der vergleich. u. experiment. Entwicklungslehre d. Wirbeltiere (1901, 1903). (G. C. C.)
- ↑ Allgemeine Physiologie, p. 53 (1895).
- ↑ Vom inwendigen Bau der Gewachse (1806).
- ↑ The Chromoplastids of the vegetable cell come under a different category of cell-inclusions; see Plants: Cytology.
- ↑ Cf. Pfeffer’s classical experiments on the physiological significance of cell-continuity in plant tissues (Über den Einfluss des Zellkerns auf die Bildung der Zellhaut, 1896). The recent work in physiology on the influence substances secreted by certain tissues and circulating in the blood-stream exert upon other and widely different tissues, should not be lost sight of in this connexion.
- ↑ The influence this protoplasmic continuity may have upon our conception of the cell as a unit of organization is referred to below (Present Position of the Cell-theory).
- ↑ A term (from κάρυον, kernel) suggested by Flemming to replace Strasburger’s hybrid term “nucleoplasm” (1882). The earlier workers, e.g. Leydig, Schultze, Brücke, de Bary, &c., restricted the term protoplasm to the cell-body—the “Cytoplasm” of Strasburger, an example still followed by O. Hertwig.
- ↑ From linum, a thread, Schwarz, 1887.
- ↑ From χρῶμα, colour, Flemming, 1879.
- ↑ The formation of pseudopodia and accompanying changes in form of Amoeba were observed as early as 1755 by Raesel von Rosenhof, who named it on this account the “little Proteus.”
- ↑ “Sur les rapports des cils vibratiles avec les centrosomes,” Archives d’anatomie microscopique (1898).
- ↑ “Über Zentralkörper in männlichen Geschlechtszellen von Schmetterlingen” (Anat. Anz. Bd. xiv., 1897). Cf. also the papers of Lenhossek (Über Flimmerzellen, 1898), Karl Peter (Das Zentrum für die Flimm- und Giesselbewegung, 1899) and Verworn (Studien zur Physiologie der Flimmerbewegung, 1899).
- ↑ Cf., however, the present writer’s interpretation of this structure in the oocyte of Antedon. Phil. Trans. Royal Soc. (1906), B. 249.
- ↑ Claude Bernard expressed the same conclusion in 1885. Rejecting both the view that vital phenomena were identical with chemico-physical phenomena, and that which regarded them as totally distinct, he suggested a third point of view: “l’élément ultime du phénomène est physique; l’arrangement est vital.”
- ↑ Many forms of response to stimulus involve no visible specialization, e.g. positive and negative heliotropism, chemiotropism, geotropism, &c., seen more especially in plants, but occurring also in the animal kingdom.
- ↑ Prominent among these are: Schleiden (1873), Fol (1873–1877), Auerbach (1874), Bütschli (1876), Strasburger (1875–1888), O. Hertwig (1875–1890), R. Hertwig (1875–1877); Flemming (1879–1891), van Beneden (1883–1887), Rabl (1889), Boveri (1887–1903).
- ↑ This distinction between the chromatic and achromatic portions of the mitotic figure is due to Flemming.
- ↑ The genesis of the spireme thread was first described by E. G. Balbiani in 1876.
- ↑ “Recherches sur la maturation de l’œuf, la fécondation et la division cellulaire” (Archives de biologie, vol. iv.).
- ↑ First discovered by Flemming in 1879 and confirmed by Retzius in 1881.
- ↑ The discovery by Hermann of the central spindle first clearly showed that two kinds of fibres must be recognized in the mitotic figure. Those of the central spindle correspond to the continuous spindle fibres of Flemming (1891) and Strasburger (1884), and the mantle fibres, i.e. half-spindle or Polstrahlen, of van Beneden (1887) and Boveri (1889–1890).
- ↑ Planter, Watasé, Griffen and others.
- ↑ e.g. Euglypha (Schewiakoff, 1888), Infusoria (R. Hertwig, 1898). So also Korschelt for Ophryotrocha, and many other cases.
- ↑ e.g. Bauer, spermatogenic cells of Ascaris univalens.
- ↑ Cf. also Watasé, Solger and Zimmermann.
- ↑ This term is due to Boveri (Zellenstudien, ii., 1888, p. 68; Jen. Zeit. xxii.), but it was intended by him to include the region of modified cytoplasm or “centrosphere” often enclosing the centrosome proper, i.e. “centriole” of Boveri.
- ↑ For outline of fertilization see article Reproduction.
- ↑ e.g. lymph and various epithelial and connective tissue cells of salamander larva (Flemming, 1891; Heidenhain, 1892); pigment cells of fishes (Solger, 1891); red blood corpuscles (Heidenhain, Eisen, 1897); and numerous other cases.
- ↑ For an interesting development of this subject see Watasé (1894). This author not only identifies the centrosome with the structures seen in lymph cells, &c., but compares it to the basal granules of ciliated cells and to the varicose swellings on the sarcostyles of striped muscle cells!
- ↑ The force of this evidence is admitted by Boveri himself. Meves, however, maintains the possibility that the numerous centrosomes appearing in the egg arise by the rapid fragmentation of a centrosome already present.
- ↑ Cf. especially the behaviour of the centrosomes in the fertilization of the egg of Pleurophyllidia (MacFarland, 1897) and that of Cerebratulus (Coe, 1901). Not only may the sperm centrosomes totally disappear before reaching the egg-nucleus, but in the latter type the definitive centrosomes appear while the last traces of the sperm asters are still visible.
- ↑ e.g. Meves; Spermatagonia of Salamandra.
- ↑ Cf. especially the artificial production of amitosis in Spirogyra; W. Pfeffer, 1899.
- ↑ Cf. Boveri, 1904, p. 13. (For Boveri’s criticism of Delage’s views, cf. Boveri, 1901 and 1902.)
- ↑ It should, however, be noted that the assumption that a particular group of characters remains always associated in a particular chromosome is one that is very difficult to reconcile with the mode of inheritance of Mendelian pairs of characters in the case of organisms with a relatively small chromosome number.
- ↑ Boveri (1902), “Fertilization of enucleated Echinus-egg fragments,” and M. Boveri (1903); by shaking the egg shortly after fertilization the sperm centrosome is prevented from dividing, and a monaster instead of a diaster results, the divided chromosomes remaining in the one nucleus.
- ↑ Cf. especially in this connexion Häcker’s paper Über die Schicksale der elterlichen und grosselterlichen Kernanteile (1902).
- ↑ Each nucleus contains a duplicate set of chromosomes, the one of maternal, the other of paternal origin, and either of these sets alone suffices for development. This is clearly shown by the experiments of Loeb (1899) and Wilson (1901) on the artificial parthenogenesis of the sea-urchin egg; and those of O. Hertwig (1889 and 1895), Delage (1899) and Winkler (1901), on the fertilization of enucleated Echinoderm eggs (Merogony, Delage). The fact that in some forms, e.g. Ascaris megalocephala var. univalens, only one chromosome is derived from each parent, originally led Boveri to conclude that all chromosomes must necessarily be physiologically equivalent.
- ↑ Über mehrpolige Mitosen als Mittel zur Analyse des Zellkerns (1902).
- ↑ Über das Auseinandergehen von Furchungs- und Gewebezellen in kalkfreien Medium (1900).
- ↑ “Entwicklungsmechanische Studien V.” (Zeit. für wiss. Zool., Bd. lv., 1892).
- ↑ See Geddes and Thomson, Sex, esp. pp. 127, 137 and 139.
- ↑ The equivalence of the germ nuclei in development is shown by the experiments on the fertilization of enucleated eggs and artificial parthenogenesis already referred to.
- ↑ O. Hertwig, 1873; but esp. van Beneden, 1883.
- ↑ Häcker, “Über die Selbstständigkeit der väterlichen und mütterlichen Kernbestandteile,” Arch. f. mikr. Anat. Bd. xlvi. (1896).
- ↑ First discovered by van Beneden (1883, 1887) for the egg of Ascaris.
- ↑ In the case of the egg the whole of the yolk stored by the “oocyte” (cell-generation immediately preceding the maturation divisions) is handed on to only one of the four resulting cells—an obvious economy. The three yolkless cells are necessarily functionless—abortive ova—and are known as the “polar bodies” (Hertwig). In spermatogenesis the maturation divisions, though bearing the same relation to reduction as in oogenesis (Platner, 1889; O. Hertwig, 1890), give rise to four functional germ-cells. The explanation of sexual differentiation given above, and that of polar body formation given here, render it needless to do more than mention the theories of Mimot (1877), van Beneden (1883) and others, by which “maturation” was regarded as removing the “male” element from the otherwise “hermaphrodite” egg.
- ↑ Weismann postulated a transverse division of the chromosomes, not a distribution of entire chromosomes; but the result as far as the reduction in the number of hereditary qualities goes is the same. The inability of the mitotic mechanism to effect the transverse division of unsplit chromosomes is pointed out by Boveri (1904).
- ↑ For an exhaustive account of reduction in Invertebrates see Korschelt and Heider, Entwicklungsgeschichte, Allgem. Teil ii. (Jena, 1903).
- ↑ Nevertheless the possibility of a pseudomitotic interpretation of maturation in Ascaris also has been maintained by O. Hertwig (1890), p. 277, Carnoy and Boveri (1904).
- ↑ The partial or even complete reconstruction of the nucleus between the heterotype and homotype division in Vertebrates makes it difficult to determine the identity of the split seen in the anaphase of the heterotype with that reappearing in the prophase of the homotype.
- ↑ e.g. Moore, 1895 (Scyllium); Flemming, 1897; Carnoy and Lebrun, 1899 (Amphibia); McGregor, 1899; Lenhossek, 1898 (mammals), and many others. So also for plants: Strasburger and Mottier, 1897; Dixon, 1896; Sargant, 1896–1897; Farmer and Moore, 1895; Gregoire, 1899; Guignard, 1899, &c.
- ↑ H. Henking (1899), T. Montgomery (1898) and F. C. Paulmeir (1899) describe the diverging bivalent halves of the tetrad as being united each by two fibres with the corresponding spindle pole. At the next division, at which the diad is resolved into its constituent univalent chromosomes, the daughter chromosomes are attached to the spindle pole each by only one fibre; the two fibres now passing to opposite poles of the spindle being the same fibres which, in the preceding mitosis, were attached to one and the same pole.
- ↑ Reference may be here made to Rosenberg’s description (1904) of the heterotype mitosis in Drosera hybrids. In the one parent (D. rotundifolia) the somatic number is 20, in the other (D. longifolia) 10; while the hybrid itself has a somatic number of 30. The reduced number in the hybrid, however, is not 15 but 20. Of these 10 are large and 10 small, the latter presumably representing the supernumerary, and hence unpaired, chromosomes of the D. rotundifolia parent.
- ↑ In their 1905 paper J. B. Farmer and J. E. S. Moore describe two successive synaptic stages (e.g. Elasmobranchs), the first during the contraction of the spireme thread, the second during the looping up of the bivalent segments. (In this paper the authors suggest the term “Meiosis” or “Meiotic phase” for the nuclear changes accompanying the two maturation divisions in plants and animals (μείωσις, reduction).
- ↑ Whitman, Jour. Morph., 1903.
- ↑ This “Precocious segregation” (Lankester, 1877) is well seen in the eggs of many Ctenophorae, Annelids, Gastropods and Nematodes. See the papers by Lillie (1901), Conklin (1902), &c., and especially Wilson on “Dentalium,” Journ. of Exp. Zool., No. 1, 1904.
- ↑ Hofmeister, de Bary, Sachs, &c.
- ↑ Loc. cit.
- ↑ Quart. Journ. Micro. Science, 1894, vol. xxxvii.