1911 Encyclopædia Britannica/Scyphomedusae
SCYPHOMEDUSAE or Acalephae, one of the two subdivisions of the Hydrozoa (q.v.), the other being the Hydromedusae (q.v.). The subclass Scyphomedusae contains a number of animals which in the adult condition are medusae or jellyfishes (see Medusa), exclusively marine in habitat and found in all seas. They are chiefly pelagic organisms, floating at or near the surface of the water, but occur also at great depths, and are sometimes fixed and sessile in habit. Many species attain a large size and by their brilliant coloration are very conspicuous objects to the mariner or traveller. In spite of the soft nature of their bodies, a number of Scyphomedusae have been found fossil; see especially Maas (7 and 12).
A scyphomedusa is distinguished from a hydromedusa chiefly by the following points. The umbrella has a lobed, indented margin, a character only seen amongst Hydromedusae in the order Narcomedusae, and it is without the characteristic velum of the Hydromedusae; hence the Scyphomedusae are sometimes termed Hydrozoa Acraspeda. The sense-organs are covered over by flaps of the umbrella margin (hence “Steganophthalmata”), and are always tentaculocysts, that is to say, reduced and modified tentacles, which bear usually both ocelli and otocysts, and are hollow. The gonads are formed in the endoderm (hence “Entocarpeae”), and the generative products are shed into the gastric cavity and pass to the exterior by way of the mouth. The development from the egg may be direct, or may take place with an alternation of generations (metagenesis), in which a non-sexual individual, the so-called scyphistoma or scyphopolyp, produces by budding the sexual medusae.
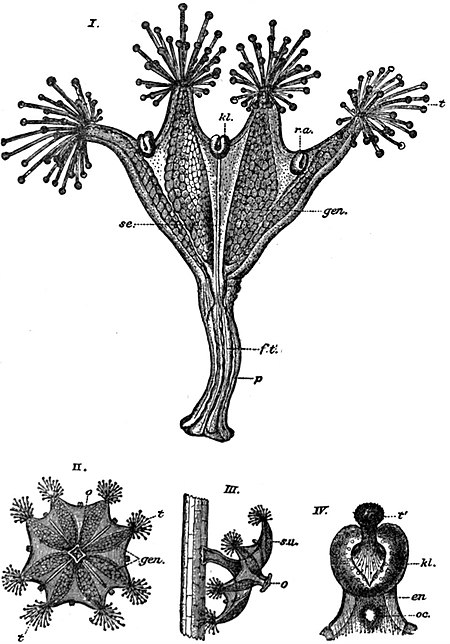
Morphology of the Scyphomedusa.—As already stated, a medusa of this order may be free-swimming or sessile in habit. Intermediate between these two types are species which have the power of temporal fixation by the exumbral surface. Such forms when undisturbed fix themselves to the bottom and rest with their mouths and tentacles uppermost. If disturbed they swim about like other medusae until a favourable opportunity presents itself for resuming the sedentary habit. A well-known example of a permanently sessile form is Lucernaria, common on the Atlantic coasts of Europe, especially in Zostera-beds, attached to the weed. It resembles in general appearance a polyp, lacking even the characteristic medusan sense-organs, which are present, however, in the allied genus Haliclystus (fig. 1), proving its medusan nature beyond all doubt.
The body-form of the Scyphomedusae varies from that of a conical or roughly cubical cap (fig. 4), to that of a shallow saucer or disk (fig. 2a). The tentacles vary in number from four, the primitive number, to a very large number, but in one suborder, the Rhizostomeae, tentacles are absent altogether (fig. 3, a). Typically the tentacles have the form of long flexible filaments, hollow or solid, implanted singly on the margin of the umbrella (fig. 3, b), but in some species they occur in groups or tufts (fig. 15), and in Lucernaria and its allies a bunch of small capitate tentacles is found on each of the eight adradial lappets of the margin (fig. 1). A true velum is absent, as already stated, but in Charybdaea (fig. 4) a structure is found termed a velarium (Ve), which is a flap hanging down from the margin of the umbrella, and which consists of a fold of the subumbral ectoderm containing endodermal canals. A true velum, such as is found in Hydromedusae, never contains endoderm.
The mouth may be a simple structure at the extremity of the manubrium, or may be four-cornered, with the corners drawn out into so-called oral arms, each of which bears on the inner side a groove continuing the angle of the mouth (fig. 2a). In some genera the oral arms are of great length, and in the suborder Rhizostomeae they undergo concrescence to form a proboscis (fig. 3, a), in such a way that the mouth becomes nearly obliterated, and is reduced to a system of fine canals opening to the exterior by small pores.
The mouth leads into the spacious stomach, which is typically four lobed (fig. 2b, v). On the floor of the stomach are borne the conspicuous gonads (ov), and also tentacle-like processes termed gastric filaments or phacellae, projecting into the cavity of the stomach. The gonads are folds of the endoderm containing generative cells, and are primitively four in number, situated interradially, but each gonad may be divided into two by the partition which separates two adjacent lobes of the stomach, that is to say, by one of the areas of concrescence between exumbral and subumbral endoderm, whence arises a condition with eight gonads which is by no means uncommon. As a rule these medusae are of separate sexes, but hermaphrodite forms are known, for example, the conspicuous British (east-Atlantic) medusa Chrysaora (fig. 3, b).
Immediately below each gonad the subumbral ectoderm is pushed in, as it were, to form a pit or deep cavity (fig. 2a, x, y) opening by a wide aperture (GP). These cavities are known as the infundibular or subgenital cavities. They serve probably for the aeration of the gonads by admitting to their vicinity water with its dissolved oxygen; they never serve as genital ducts, since the generative products are always dehisced into the stomach and pass out by the mouth. In some genera, for instance, Cyanea and its allies the gonad as a whole protrudes through the subgenital cavity as if it had undergone a hernia, and hangs down in the subumbral space as if suspended by a mesentery (fig. 15). Usually the four subgenital cavities are distinct from each other (so-called tetrademnic condition), but in many Rhizostomeae, for example, Crambessa, the subgenital cavities join together under the subumbral floor of the stomach (so-called monodemnic condition) and coalesce to form a so-called subgenital portico placed on the oral side of the stomach, opening by four interradial apertures between the oral arms, that is to say, by the four primitive apertures of the subgenital pits. In Nausithoë subgenital pits are absent altogether, and the same condition may be found in Charybdaeidae.
The gastroyascular system shows every degree of complexity from a very primitive to a highly elaborate type of structure. Taking as a starting-point the wide archenteric cavity which the medusa inherits primitively from the antecedent actinula-stage (see article Medusa), we find, in such a form as Tessera, four interradial areas of concrescence between the exumbral and subumbral layers of endoderm, four so-called septal nodes or “cathammata,” subdividing the stomach into four wide, radially situated pouches which communicate with each other beyond the septal nodes by wide apertures constituting what is termed by courtesy a ring-canal. In other cases the areas of concrescence may extend as far as the margin of the umbrella, so that the lobes of the stomach are completely separated from one another, as in Charybdaea (fig. 4), where there are four gastric pouches communicating with the central stomach by four so-called gastric ostia (fig. 4). A similar condition is seen in Pelagia, where the number of gastric pouches is increased to sixteen. In forms such as Lucernaria and Charybdaea, in which the umbrella is of deep form and the stomach-cavity consequently of great extent in the vertical direction, the concrescence-areas or septal nodes are drawn out into vertical partitions or taeniolae (fig. 4, L.o.c.), resembling in their anatomical relations the mesenteries of the Anthopolyp. The phacellae are carried on the edges of the taeniolae (fig. 4, Gh). Finally in the majority of Scyphomedusae the primitively simple concrescence-areas become increased in number and in extent, so that radial canals, ring-canals, &c., can be distinguished in addition to stomach-pouches. Thus in Aurelia (figs. 2a and 2b), to take a familiar example, the digestive tract begins with the mouth, of which the four corners are prolonged into the four long oral arms, perradial in position. The mouth leads into the spacious stomach containing the four conspicuous horse-shoe-shaped gonads (ov) marking four stomach-pouches, which, however, are interradial in position. From the stomach or its pouches arise sixteen radial canals, four perradial, four interradial and eight adradial (fig. 2b). The perradial and interradial canals consist of a main stem giving off branches, and both stem and branches reach to the marginal ring-canal, the main stem ending in one of the eight tentaculocysts, which are lodged in the notches between the lobes of the umbrellar margin. The adradial canals are unbranched and run to the middle point of one of the marginal lobes. The system of canals shows great variation even in the same species.
The muscular system of the Scyphomedusae is developed on the subumbral surface as a system of circularly disposed fibres which by their contraction make the umbrella more concave and diminish its cavity. The circular muscles usually form two chief portions, a peripheral wreath-muscle (Kranzmuskel), subdivided into four, eight or sixteen areas, and an oral ring-muscle round the mouth. Endodermal muscles are found in the phacellae, and in such forms as Lucernaria, longitudinal (vertical) muscular tracts or bands are found in the taeniolae, which, according to some authorities, are of endodermal origin, but which, according to recent observations, are formed in the walls of the infundibular cavities, and are therefore of ectodermal origin.

|
|
Fig. 3.—Scyphomedusae. a, Rhizostoma pulmo; b, Chrysaora hysoscella. |
The nervous system consists as in Hydromedusae of a diffuse plexus beneath the ectoderm, concentrated in certain places to form a central nervous system. In these medusae, however, the central nervous system does not form continuous rings, but occurs as four or eight separate concentrations at the margin of the umbrella, centred each round one of the sense-organs (tentaculocysts). Each nerve-centre controls its own antimere or segment of the body, receiving sensory impressions from the tentaculocyst and innervating its special subdivision of the muscular system. The separate nerve-centres are, as a rule, placed in communication only by the general nerve-plexus, but in Charybdaea there is a zigzag marginal nerve connecting them up.
The sense-organs of the Scyphomedusae are on the whole of a very uniform type. They are always tentaculocysts, as already stated, and they always have hollow axis, unlike the tentaculocysts of Hydromedusae, in which group these organs, when they do occur (as in Trachylinae) are always solid. Two types of tentaculocyst must be distinguished, the one occurring only in the order Stauromedusae, the other in all orders of the group. The second and commoner type is known as a rhopalium (fig. 6) and consists of a short, hollow rod, the wall of which is composed of the two body-layers, ectoderm and endoderm, enclosing a cavity continuous with that of the gastrovascular system. At the apex of the rhopalium the endoderm is greatly thickened and consists of concrement-cells secreting otoliths (Con). The more proximal portion of the rhopalium usually bears one or more ocelli (oc). The rhopalia are lodged in the notches between the marginal lobes of the umbrella, and each rhopalium is covered over by a little protecting flap or lappet. On the external (i.e. exumbral) face of the lappet there is frequently a patch of sensory ciliated epithelium regarded as olfactory in function and termed the olfactory pit (fig. 6, A). Each rhopalium is a centre round which, as already stated, nervous tissue is concentrated.
The otoliths vary considerably in number and size. In Aurelia there are found numerous otoliths arranged irregularly. In Charybdaea (fig. 7, otol) the otoliths are larger but fewer in number and have a definite arrangement. In Nausithoë a single large otolith is found.

|
|
Fig. 5.—Scattered Nerve Ganglion Cells. c, From the subumbrella of Aurelia aurita. (After Schäfer.) |
The ocelli vary greatly both as regards number and complexity of structure. In some genera they are absent, as, for instance, in Pelagia, Cyanea and Rhizostoma. In Aurelia there are two on each rhopalium, a simple ocellus on the exumbral side, and a cupped ocellus on the subumbral side (not present in young individuals). In Charybdaea there are no less than six ocelli on each of the four rhopalia (fig. 7); on the exumbral aspect there are two median ocelli (oc¹, oc²), a distal and a proximal, each of them a vesiculate ocellus with a lens, and on the sides of the rhopalium are two pairs of ocelli without lenses (oc.l); sometimes also an additional seventh ocellus occurs, a pit-like structure without a lens either between the two median ocelli, or placed asymmetrically near the median proximal ocellus.
The ocelli consist, as in Hydromedusae, of two kinds of elements: (1) visual cells, sensory ectodermal cells, which may develop terminal visual cones; (2) pigment-cells, usually ectodermal, but in one known instance endodermal. The simplest type of ocellus is exemplified by the exumbral ocellus of Aurelia, a simple patch of pigment-cells interspersed with visual cells, the whole on a level with the remaining ectodermal epithelium. In the next stage of complication, seen in the supernumerary (seventh) ocellus of Charybdaea, the patch of pigmented and sensory epithelium is pushed in to form a little pit, in the interior of which the pigment-cells secrete a gelatinous substance forming a rudimentary vitreous body. As a further advance, the pit becomes widened out into a cup, as in the lateral ocelli of Charybdaea. The culminating stage of evolution is seen in the median ocelli of Charybdaea (fig. 8); the primitively open cup has now closed over to form a vesicle lying beneath the ectoderm; the outer wall of the vesicle becomes thickened to form a cellular lens (l), while the proximal wall consists of sensory and pigmented cells and forms a retina. In this way the ocellus becomes a true eye, very similar in plan to the eyes of Gastropods and other molluscs. The ectoderm continued over the optic vesicle forms a transparent cornea (fig. 8, c) (better perhaps termed a conjunctiva), below which the spherical lens projects into the optic vesicle, imbedded in the vitreous humour (v.b) which fills it; the retina (r) consists of visual cells with long cones (fig. 9) alternating with pigment-cells. The high development of the eyes of Charybdaea is very remarkable, and so is their close resemblance to the eyes found in other groups of the animal kingdom, with which they can have no genetic relation. Highly developed eyes, with ectodermal pigment and lens, are found also on the rhopalia of Paraphyllina (Maas [8]).
The subumbral ocellus of Aurelia is found to be of the inverted type, with the visual cones turned away from the light, as in Tiaropsis amongst Hydromedusae, and here also the pigment is furnished by the endoderm, forming a cup into which the ectodermal visual cells project (Schewiakoff [13]).
In the Stauromedusae tentaculocysts are either absent altogether, as in Lucernaria, or represented by peculiar structures termed “colletocystophores” or “marginal anchors” (fig. 1, IV.). Each such body has a basal hollow portion (en) surmounted by a glandular cushion (kl), from the centre of which projects a small, solid, club-shaped process or tentacle (t′). The basal portion bears an ocellus (oc) of simple structure, The distal club corresponds to the crystal-sac of an ordinary rhopalium, but bears a battery of nematocysts in place of the otoliths. These organs are said to be used for purposes of adherence rather than to have the function of sense organs. The histological structure of the Scyphomedusae is in the main similar to that of the Hydromedusae (q.v.), but the mesogloea is more abundantly developed in the free-swimming forms, and contains special mesogloeal corpuscles, derived by immigration from the ectoderm, an generally occurring in the form of stellate or bipolar cells.
Development of the Scyphomedusae.—No adult Scyphomedusae are known to reproduce themselves by budding or by any method other than the sexual one. The course of the development in this group is best made clear by taking as a type Aurelia, which, together with certain other common genera, such as Chrysaora and Cotylorhiza, has been studied in detail. Unfortunately the statements concerning some points are very contradictory.
The ova pass out of the mouth and are fertilized externally. In some cases the ova, after leaving the mouth, are lodged in the oral arms, and undergo the earliest phases of their development in this situation, accumulating in the grooves that continue the angles of the mouth, and bulging the wall of the groove into sacs or pockets.
The ovum undergoes total cleavage, giving rise to a bastula which forms a gastrula (fig. 10, A) by invagination (see article Hydrozoa) This is a type of germ-layer formation never found in the Hydromedusae, though of universal occurrence in all groups of animals above the Coelentera. We may regard it as a form of unipolar immigration in which the immigrating cells pass into the interior in a connected epithelial layer, instead of going in singly and independently. The embryo is set free as a planula larva (fig. 10, B) in the gastrula stage, and the orifice of invagination or blastopore, which persists, is situated at the hinder pole. After a time the planula fixes itself by the anterior pole, with the blastopore uppermost. The larva after fixation changes into a polyp-like organism termed a scyphistoma or scyphopolyp (fig. 10, C, D). The body becomes in shape like a vase or urn attached by a narrow stalk, round which a chitinous membrane is secreted. From the edges of the vase the four primary tentacles grow out, each a slender filament with a solid endodermal axis. The tentacles border a broad, flattened peristome, from the centre of which arises the hypostome with the mouth at its extremity; the hypostome is at first low, but soon becomes a projecting, chimney-like tube. It has been sought to prove that the interior of the hypostome is lined by ectoderm, so as to form a stomodaeum or ectodermal oesophagus similar to that of the Anthozoa, but this has been disproved by the most recent investigations of Hein (4) and Friedemann (3), who have shown that the mouth at the extremity of the hypostome represents the persistent blastopore of the gastrula stage.
The internal gastric cavity of the scyphistoma is not a simple space as in the hydropolyp, but is subdivided by four ridges or taeniolae, arising one in each interradius (fig. 11, B). Each taeniola is similar in its anatomical relations to the similarly named structures in Haliclystus (fig. 1), and becomes perforated in the same way at its outer side by a “septal ostium,” forming as it were the rudiment of a ring-canal. Each taeniola bears a strongly developed longitudinal muscle-band, stated by Claus and Chun to be developed from the endoderm, like the retractor muscles of the anthopolyp, but by other investigators it is affirmed that each retractor muscle of the scyphistoma arises from the lining of a funnel-shaped ectodermal ingrowth (“Septaltrichter”) growing down from the peristome inside each taeniola, in a manner similar to the infundibular cavities of Lucernaria, which in their turn are homologous with the subgenital cavities of other Scyphomedusae. It is asserted, however, by Friedemann (3), a recent investigator of the subject, that the infundibular cavities appear late in the scyphistoma and have no relation either to the septal muscles or to the subgenital cavities of the adult. The muscle-bands are very contractile, rendering the scyphistoma one of the most difficult of all organisms to preserve in an expanded condition. By their contraction the muscles of the taeniolae drag the hypostome down and so produce the appearances which have been interpreted as a stomodaeal invagination.
As the scyphistoma grows the tentacles increase in number, four interradial and eight adradial being formed in addition to the four primary perradial tentacles (fig. 11, A, B, C). The animal may produce its like by lateral budding, or by budding from a basal stolon. The scyphistoma of Nausithoë forms a branching network which grows in the sponge Esperella and forms the colonial polypoid organism named by Schulze Spongicola fistularis, by Allman Stephanoscyphus mirabilis. Sooner or later, however, the scyphistoma produces free medusae by a process of transverse fission termed strobilization. In the simplest case one medusa, or at least one at a time, is produced in this way (monodisk strobilization); a circular furrow cuts off the upper, tentacle-bearing portion from the lower half of the scyphistoma (fig. 11, D, and fig. 12), and the upper part becomes detached and swims away, while the base regenerates a new crown. In most cases, however, many such furrows are formed (polydisk strobilization), so that the animal comes to resemble a pile of saucers one above the other (fig. 12). The uppermost saucers of the pile become detached successively and swim off. In this state the scyphistoma is termed a strobila.
The medusae produced by strobilization of the scyphistoma are of a peculiar type termed Ephyrae (fig. 11, E, F). As preparations for their formation the margin of the peristome of the scyphistoma grows out into eight lobes, four perradial, four interradial. The sixteen tentacles of the scyphistoma disappear, and in the place of the four perradial and four interradial tentacles, the eight tentaculocysts of the adult are formed as outgrowths of the subumbral margin, independently of the tentacles of the scyphistoma (Friedemann). The septal ostia become widened and the gastral cavity flattened, whereby the taeniolae become comparatively shallow columns, similar to the septal nodes or cathammata of other forms.[1]
The ephyra has a flat, disk-shaped body, with eight marginal lobes (four perradial, four interradial); a tentaculocyst is lodged in a deep notch at the apex of each lobe. Four groups of phacellae indicate the four interradii. The stomach has sixteen marginal pouches and the general anatomical structure recalls that of Pelagia. As the ephyra grows in size it gradually takes on the form and structure of the young medusa. The adradial regions grow (fig. 11, F) so as to change the star-like contour into one more evenly circular, the tentacles grow out, and the various parts become complicated and take on the structure of the adult medusa.
The course of development sketched out above is that which is typical of the higher forms of Scyphomedusae, and is by no means to be regarded as the most primitive type of development. The complicated alternation of generations seen in such a form as Aurelia does not occur in the more primitive genera. Thus in Pelagia the scyphistoma-stage is free-swimming and changes directly into the ephyra, which in its turn grows into the adult form. On the other hand, such a form as Lucernaria or Haliclystus may be regarded simply as a scyphistoma which has become adult and mature. The comparison of the metagenetic type of development, such as that of Aurelia, with the more primitive genera of Scyphomedusae, indicates clearly that the scyphistoma and ephyra are recapitulative larval stages which are represented by the adult forms of primitive genera, making such allowances as are necessary when comparing adult and larval forms. The metagenesis has arisen through the scyphistoma-larva acquiring the power of larval proliferation by budding. A similar origin for metagenesis has been discussed under the Hydromedusae (q.v.).
The above comparison further indicates that the scyphistoma should not be regarded as a polyp but rather as a medusoid organism. The only certain criterion of a medusa-individual is the presence of definite sense-organs, but in cases where the organism is much reduced, this criterion may fail us, as it does in the genus Lucernaria. Nevertheless a comparison between Lucernaria and its close ally Haliclystus shows clearly that the absence of sense-organs in the former is the result of secondary reduction, so that a true medusa may lose its most characteristic feature. Hence the absence of sense-organs in the scyphistoma does not necessarily disprove its medusoid character, while its anatomical structure resembles that of a simple scyphomedusa, such as Lucernaria, rather than that of a polyp.
Affinities of the Scyphomedusae.—By some authorities the Scyphomedusae have been removed from the Hydrozoa and united with the Anthozoa in a common group termed Scyphozoa. The diagnostic features of the class Scyphozoa thus constituted are supposed to be (1) an ectodermal oesophagus or stomodaeum, (2) a gastric cavity subdivided by mesenteries, (3) gonads formed in the endoderm. It appears, however, that the first of these characters is non-existent, and that the so-called mesenteries are simply the concrescence-areas found in all medusae. There remains only the third feature, the endodermal gonads, as an argument for uniting the Scyphomedusae with the Anthozoa, against which must be set all the peculiarities of medusan organization in which the Scyphomedusae resemble the Hydromedusae. The fact that the Scyphomedusae have a number of well-marked peculiarities of form and structure is not incompatible with placing them in the Hydrozoa as a distinct sub-class, contrasting sharply in many ways with the Hydromedusae.
Classification of the Scyphomedusae
Order I. Cubomedusae or Charybdaeida.—Medusae more or less cubical in form, with four perradial rhopalia alternating with four interradial tentacles or groups of tentacles; oral arms short; stomach a wide cavity bearing four interradial groups of phacellae and giving off four broad perradial pouches completely separated from each other by four interradial septa (i.e. ring-canal absent); gonads divided each into two by the septa, hence eight in number; subgenital pits small or absent.
This order stands very much apart from the other orders of the Scyphomedusae. It has been proposed by Maas to divide the entire subclass Acraspeda into A, Charybdaeida and B, Acraspeda typica. The Charybdaeida comprise three families:—
1. Charybdaeidae.—With four interradial tentacles. Charybdaea marsupial is (fig. 4) is a familiar Mediterranean medusa; the wonderful development of the sense-organs in this genus has already been described (figs. 7-9). The species of Charybdaea are stated to be quick and active in their movements and to be voracious feeders.
2. Chirodropidae.—With four interradial groups of tentacles. Chirodropus.
3. Tripedaliidae.—With four interradial groups of tentacles, three in each group. Tripedalia.
Order II. Stauromedusae or Lucernarida.—Medusae of deep pyramidal form, often sessile, attached by a stalk developed from the centre of the exumbral surface; rhopalia absent or represented by colletocystophores. Four families:—
1. Lucernaridae.—Sessile, stalked, with capitate tentacles arranged in groups on eight projecting marginal lobes. Eight gonads. Lucernaria, without, and Haliclystus (fig. 1) with colletocystophores, are two well-known genera.
2. Tesseridae.—Free, with eight or more tentacles, without tentaculocysts. Tessera, &c.
3. Depastridae.—Sessile, stalked, with eight shallow marginal lobes bearing one or more rows of tentacles; without tentaculocysts; with four gonads. Depastrum is a British genus.
4. Stenoscyphidae.—Sessile, with the margin undivided; with eight colletocystophores and eight adradial groups of capitate tentacles. Stenoscyphus inabai, from Japan.
Order III. Coronata.—Free medusae with rhopalia of the normal type; the exumbrella is divided by a circular, so-called coronal groove, into two parts, a central portion, which is conical, thimble-shaped, or domed in form, and a peripheral portion, the pedal zone, which bears the marginal lobes, tentacles and rhopalia; the pedal zone is subdivided into areas termed pedalia, from each of which arises a tentacle or rhopalium in the interspace between two adjacent lobes of the margin. The order contains the following families:—
1. Periphyllidae.—With sixteen marginal lobes, four rhopalia and twelve tentacles; the rhopalia are interradial. Periphylla (fig. 13), a widely distributed deep-sea genus.
2. Paraphyllinidae.—With sixteen marginal lobes, four rhopalia and twelve tentacles; the rhopalia are perradial in position, corresponding to the angles of the stomach. Paraphyllina recent; Paraphyllites fossil [see Maas (8 and 12)].
3. Atorellidae.—With twelve marginal lobes, six rhopalia and six tentacles. Atorella.
4. Pericolpidae.—With eight marginal lobes, four rhopalia and four tentacles. Pericolpa.
5. Collaspidae (Atollidae).—With sixteen or thirty-two rhopalia, marginal lobes and tentacles often very numerous. Atolla (fig. 14) is a well-known deep-sea genus.
6. Ephyropsidae.—With Sixteen marginal lobes, eight rhopalia and eight tentacles. Nausithoë, a small medusa of world-wide distribution, is the type of the subfamily Nausithoidae; the subfamily Linergidae includes the genera Linerges, &c., medusae confined to tropical seas. By Maas and others the Nausithoidae and Linergidae are ranked as independent families.
Order IV. Discophora.—Medusæ with umbrella flattened or disk-like, without coronal groove; lips always prolonged into long oral arms. The most prolific and dominant group of the Scyphomedusae, containing two suborders; the Semaeostomae, in which the oral arms remain separate, and the Rhizostomeae, in which the oral arms become fused together to form a proboscis. Nine families, three of Semaeostomeae, six of Rhizostomeae:—
|
|
Modified from a coloured plate in Prince of Monaco's series. |
|
Fig. 14.—Atolla bairdi. After O. Maas. |
1. Pelagiidae.—Semaeostomeae with wide gastric pouches not united by a ring-canal. Pelagia, an oceanic genus with direct development. Chrysaora (fig. 3b), a common British medusa, with a scyphistoma stage and alternation of generations. Dactylometra, a common American medusa of the Atlantic shores, differs from Chrysaora in small points.
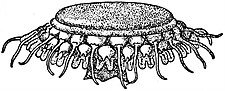
2. Cyaneidae.—Semaeostomeae with sixteen gastric pouches sending off canals to the margin not united by a ring-canal; tentacles in bunches on the margin. Cyanea (fig. 15), represented in the British fauna by two species.

|
|
After E. Haeckel, from System der Medusen, by permission of Gustav Fischer. |
|
Fig. 15.—Cyanea (Desmonema) anasethe, about two-thirds life-size. |
3. Ulmaridae.—Semaeostomeae with gastric pouches relatively small, sending off branching canals to the margin, where they are united by a ring-canal. Ulmaris, from the South Atlantic, has only eight adradial tentacles. Aurelia (fig. 2), with numerous marginal tentacles, is one of the commonest and most familiar of jellyfishes.
4. Cassiopeidae.—Rhizostomeae with subumbral musculature arranged in feather-like arcades (Arcadomyaria, Maas); oral arms pinnate. Cassiopeia.
5. Cepheidae.—Rhizostomeae with subumbral musculature in radial tracts (Radiomyaria, Maas); oral arms bifid. Cephea, Cotylorhiza.
6. Rhizostomatidae (Pilemidae).—Rhizostomeae with subumbral musculature in circular bands (Cyclomyaria); oral arms bifid or very complicated; sixteen radial canals. Rhizostoma (Pilema) is a very common genus (fig. 3a).
7, 8, 9. The families Lychnorhizidae, Leptobrachidae and Catostylidae resemble the preceding in the arrangement of the musculature. In Lychnorhizidae only eight of the sixteen radial canals reach the ring-canal; the genus Crambessa is the best-known representative of the family. In the other two families there are eight radial canals, and between them a network of canals with many openings into the ring-canal.
Bibliography. 1. E. T. Browne, “Variation in Aurelia aurita,” Biometrika, i. (1901), pp. 90-108, 3 figs.; 2. “Scyphomedusae,” Fauna and Geogr. Maldives and Laccadives, ii., suppl. i. (1905), pp. 958-971, pl. xciv.; 3. O. Friedemann, “Untersuchungen über die postembryonale Entwicklung von Aurelia aurita,” Zeitschr. f. wiss. Zool. lxxi. (1902), pp. 227-266, pls. xii. xiii., 3 text-figs.; 4. W. Hein, “Untersuchungen über die Entwicklung von Aurelia aurita,” Zeitschr. wiss. Zool. lxvii. (1900), pp. 401-438, pls. xxiv. xxv., 5 text-figs.; 5. K. Kishinouye, “Some New Scyphomedusae of Japan,” Journ. Coll. Sci. Tokyo, xvii., No. 7 (1902), 17 pp., 2 pls.; 6. O. Maas, “Die Medusen” (Albatross Expedition), Mem. Mus. Comp. Zool. Harvard Coll. xxiii. 1 (1897), 92 pp., 15 pls., with explanations; 7. “Über Medusen aus dem Solenhofer Schiefer und der unteren Kreide der Karpathen,” Palaeontographica, xlviii. (1902), pp. 297-322, pls. xxii. xxiii., with explanations, and 9 text-figs.; 8. “Die Scyphomedusen der Siboga-Expedition,” Uitkomst. Siboga-Expeditie, xi. (1903), 91 pp., 12 pls., with explanations; “Méduses,” Result. Camp. Sci. Albert, Monaco, xxviii. (1904), 71 pp., 6 pls., with explanations; 10. “Médusen,” in Résultats du S. Y. Belgica (1906), 32 pp., 3 pls.; 11. “Die arktischen Medusen,” Fauna Arctica, iv. (1906), pp. 479-526; 12. O. Maas, “Über eine neue Medusengattung aus dem lithographischen Schiefer,” N.J.B. Mineral Geol. Palaeontol. (1906), ii. pp. 90-99, 4 text figs.; 13. W. Schewiakoff, “Beiträge zur Kenntnis des Acalephenauges,” Morph. Jahrb. xv. (1889), pp. 21-60 3 pls.; 14. E. Vanhöffen, “Die Akalephen der Plankton Expedition,” Ergebn. d. Plankt.-Exp. (1892), 28 pp., 5 pls.; 15. “Die acraspeden Medusen der deutschen Tiefsee-Expedition,” Deutsch. Tiefsee-Exped. “Valdivia,” iii. (1902), pp. 1-15, 8 pls. See also the general works cited in the article Hydrozoa and the bibliographies given in them. (E. A. M.)
- ↑ The four primitive interradial cathammata disappear in the fully formed ephyra and become replaced by sixteen subradial concrescence-areas without any ostia or ring-canal at the margin.