somewhat after the method of Newlands, but this table could be considered in no sense an advance upon the table of his English contemporary. It was not so much a periodic table as a summary of the grouping of the elements in more or less natural groups. Meyer, indeed, made his earlier table the basis of his later work, but these subsequent amendments to the table were made after the publication of Mendeléeff's first table and show clearly the influence of his work.
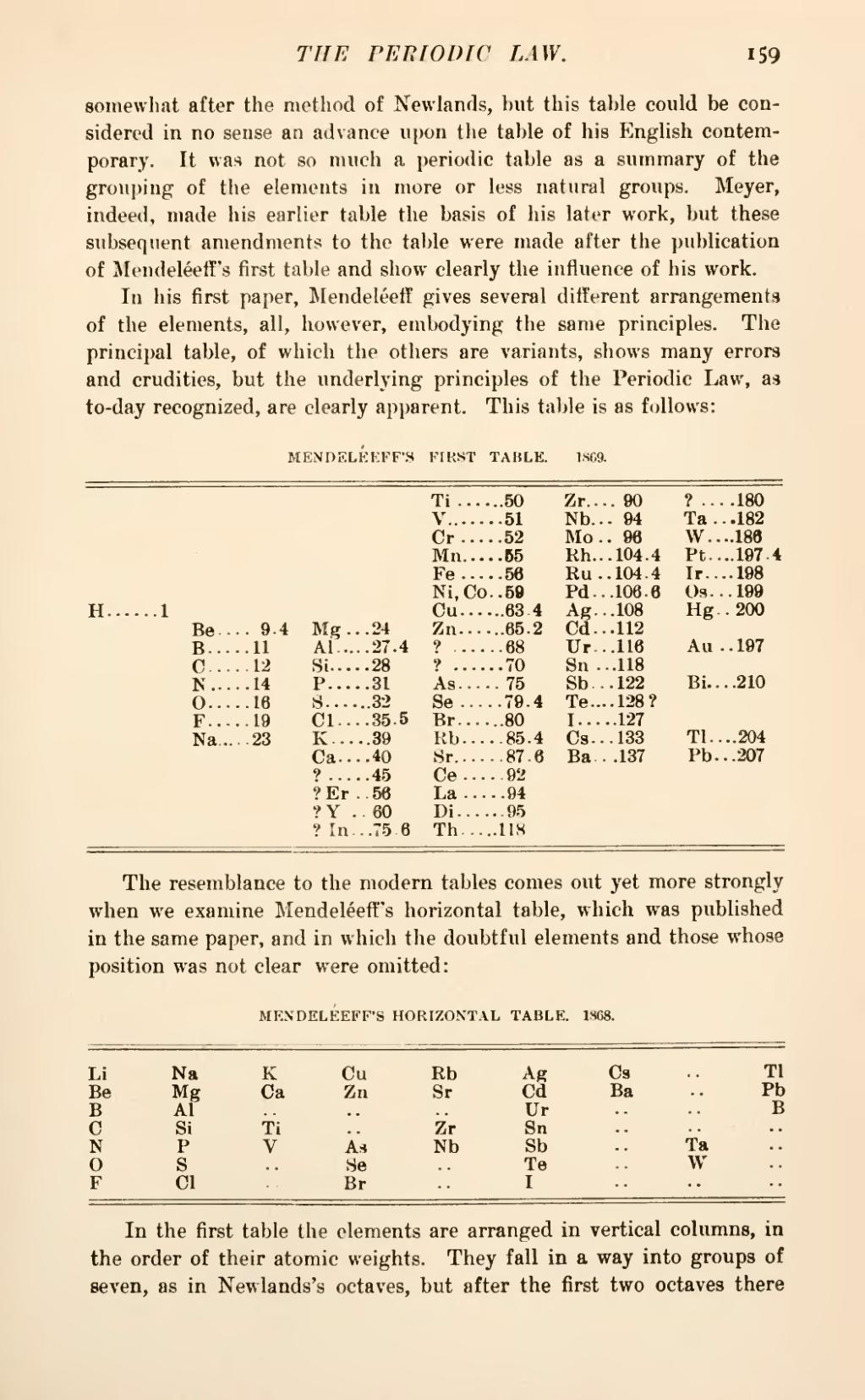
In his first paper, Mendeléeff; gives several different arrangements of the elements, all, however, embodying the same principles. The principal table, of which the others are variants, shows many errors and crudities, but the underlying principles of the Periodic Law, as to-day recognized, are clearly apparent. This table is as follows:
MENDELÉEFF'S FIRST TABLE. 1809.
The resemblance to the modern tables comes out yet more strongly when we examine Mendeléeff's horizontal table, which was published in the same paper, and in which the doubtful elements and those whose position was not clear were omitted:
MENDELÉEFF'S HORIZONTAL TABLE. 1868.
In the first table the elements are arranged in vertical columns, in the order of their atomic weights. They fall in a way into groups of seven, as in Newlands's octaves, but after the first two octaves there