The New International Encyclopædia/Iron and Steel, Metallurgy of
IRON AND STEEL, Metallurgy of. In some cases the ores of iron can at once be treated in the blast-furnace without preparation of any kind. In all cases the preliminary treatment must be simple and cheap, in order to be commercially practicable. The common methods of preparing iron ore for the blast-furnace are the following: Sorting and sizing consists in roughly separating the ore from the barren rock and other fragmentary impurities, and then grading it into lumps of similar size to promote regularity in smelting. Washing consists in removing clay and other earthy matter from the ore by means of water; both hand washing and mechanical washers are employed. Magnetic concentration consists in passing naturally fine ores, or ores made fine by crushing, through so-called separators, in which the ore is drawn from the non-ferrous gangue by means of magnetized drums or other mechanism. Weathering consists in stacking the ore in heaps to remove shale and sulphur, which crumble or wash away under the action of the elements. Calcination consists in treating the ores to drive off volatile matters, usually water, carbon dioxide, sulphur, and carbonaceous matter, or to oxidize the ore. Calcination may be accomplished by washing the ore in open heaps or by heating it in calcining kilns. Sometimes a combination of two or more of the methods of preparation previously described is employed.

|
|
Fig. 1. VERTICAL SECTION OF A BLAST-FURNACE. |
Cast Iron. As stated under Iron, cast iron was first produced in Germany. Previous to about 1350 the highest temperatures obtainable in the blast-furnace had been barely sufficient to produce a pasty bloom of iron which then had to be hammered to remove the cinder and forged into shape. During the early part of the fourteenth century, however, German iron-makers, in their endeavors to reduce the cost of manufacture, began to build blast-furnaces of gradually increasing size, from which, by allowing the metal to remain longer in contact with the fuel, iron was obtained in a molten condition capable of being readily cast into any desired shape. At once the founding of iron became an important art. It was not long, however, before the fact was established that by further treatment the cast iron would be transformed into wrought iron. To-day the production of cast iron in the blast-furnace is the first step in the manufacture of iron and steel.
With the discovery of cast iron and the introduction of its manufacture into England, the British iron trade, which had lain dormant, began to revive, and at the end of the sixteenth century it had assumed very considerable proportions. At first charcoal was the fuel used in the blast-furnace, and this fact resulted in such depletion of the timber-supply that several acts of Parliament were passed between 1558 and 1584 restricting the number and position of iron-works and prohibiting the construction of new works in certain districts. A decline in iron manufacture was the natural result.
Naturally, also, attention was turned to the possibility of some substitute for charcoal. This was discovered about 1619 by Dud Dudley, who produced coke from pit coal, and successfully used it for smelting iron in the blast-furnace. The substitute did not gain favor, for various reasons, which it is needless to mention further than to say that they were not lack of success in the use of coke.
During all of the seventeenth century charcoal remained the almost universal fuel for blast-furnaces. These furnaces were small and widely scattered: they had a capacity of about 20 tons of cast iron per week each, were built of masonry, and the blast was supplied by bellows operated by water-power. In 1713 Abraham Darby, of Coalbrook Dale, in Shropshire, revived the use of coke fuel for blast-furnaces, and after much labor succeeded about 1740 in making a success of it. The use of coke now spread rapidly; and this, with the development of the steam-engine for blowing purposes and for supplying power to mills and forges, gave an enormous impetus to iron manufacture.
No further improvement in blast-furnace practice occurred until 1828, when J. B. Neilson, an Englishman, brought forward a proposition to heat the air for the blast. The greatly increased output which resulted from the use of the hot blast set on foot a series of improvements in blast-furnace construction. These improvements took the form of increased dimensions and capacity of the furnace and of the use of an increased number of tuyeres; the stove for heating the blast air was improved, more efficient and powerful blowing engines were employed, apparatus for hoisting the ores, fluxes, and fuel, and for charging them into the furnace were introduced, and a great variety of minor improvements were made. Until 1880 British furnaces led the world in size and output; but about this time American iron-makers began to take the lead in these respects, and have maintained it ever since. A few figures selected at random will show the progress of growth in blast-furnace dimensions, capacity, and output: 1855 to 1861, height 30 feet, capacity 2000 cubic feet, output 200 tons weekly; 1882 to 1885, height 70 feet, capacity 8200 cubic feet, output 800 tons weekly; 1892 to 1895, height 90 feet, capacity 18,200 cubic feet, output 2500 tons weekly; 1900, height 100 feet, capacity 24,000 cubic feet, output 600 tons every 24 hours. At this point it may be noted that the first iron-furnace in America was a bloomery erected in Virginia in 1619, and the first blast-furnace with a forced blast was built about 1714 in the same State. Shortly after the Revolutionary War numbers of charcoal-furnaces were working. From this time on the growth of iron and steel manufacture in America was rapid, until in 1890 the United States took first place among the iron-working nations of the world.
The internal shape and general construction of a modern American blast-furnace are shown by Fig. 1. As will be seen, the structure resembles in shape the common glass lamp-chimney. The cylindrical portion at the bottom is called the hearth, the bellying portion next above is the boshes, and the conical portion above forms the stack. The general construction is a masonry lining within a steel shell. To keep the hearth cool it is surrounded by a water-jacket. Air is supplied by tuyeres drawing from a circular blast-main. At the top there is a funnel-shaped charging hopper closed by a conical door and suitable exits for the furnace gases. These gases pass down through metal pipes to the stoves for heating the blast. Blast pressure is supplied by blowing engines which draw in cold air and discharge it through the hot-blast stove, whence it passes into the blast-mains and then through the tuyeres into the furnace. The furnace is charged by means of a car carrying a suspended skip, which is hauled up an incline to the top of the furnace and then discharged into the charging hopper. While the foregoing description applies to a particular furnace, it will serve in a general way for all blast-furnaces.
In operation a blast-furnace is charged at the top with approximately alternate layers of ore, limestone, and fuel, usually coke. At the bottom of the furnace is introduced a current of hot air through the tuyeres. The materials introduced into the furnace, therefore, form two currents moving in opposite directions, one current being composed of hot gases and the other of solid substances. The chemical reactions which take place between these two currents result in the production of molten iron, molten slag, and gases. These reactions are of very complex nature, and can only be indicated in a general way. When the highly heated blast enters the furnace the carbon of the fuel burns with the oxygen of the air to form carbon dioxide; this, at the high temperature prevailing in the hearth, is almost immediately dissociated, and the liberated oxygen combines with more carbon to produce carbon monoxide. Carbon monoxide, being a powerful reducing agent, takes oxygen from the ore to produce carbon dioxide. When the iron ore is charged into the furnace it at first suffers no chemical change, but gradually absorbs heat until at a temperature of about 200° C. it begins slowly to lose oxygen. As the temperature rises and the materials descend in the furnace the reduction becomes more rapid until at 600° C. it is very rapid. At this temperature also the limestone decomposes, forming quicklime and liberating carbon dioxide, part of which takes up carbon from the fuel-producing carbon monoxide. When the charge has passed through about 30 feet of the stack it has been deoxidized, and consists of lumps of spongy iron, side by side with pieces of coke and quicklime. The descent of the charge continues for 30 or 40 feet without much change, until a temperature sufficient for the formation of slag has been reached, when the silica and other bases combine with the lime to produce slag. The charge then melts and runs down into the hearth, and collects below the level of the tuyeres in two layers, one of molten iron at the bottom and the other of molten slag on top.
The next step is to tap the furnace and draw off, first the molten slag, and then the molten metal. Generally the slag is run to waste, but sometimes it is preserved and submitted to treatment which permits its utilization. Some of these uses are road metal, railway ballast, slag bricks, slag wool, and hydraulic cement. Bricks are made by casting the molten slag in molds. To produce slag wool the molten slag is blown by a jet of steam, which produces small globules, to each of which is attached a long thin filament. (For the utilization of slag in cement-making, see Cement.) Slag, whatever use may be made of it, is only an incidental product; the essential thing is to secure the molten iron in suitable form for use. To do this the molten iron is run into molds, which produce east ingots or bars called pigs.
Formerly the casting was performed entirely in sand molds formed in the casting floor; but at present the largest and best equipped blast-furnace plants employ casting machines. In casting in sand molds, these molds of trough shape are formed thickly over the casting floor; the iron from the furnace flows into a large main channel, thence into side channels, and thence into the individual molds, until a part or all of them are filled with molten metal. This is allowed to solidify, and then the pigs are removed and the molds prepared for another set of pigs. In pig-casting machines two endless chains carry a series of pressed-steel molds, which travel at slow speed past a trough terminating in two mouths. The molten iron from the furnace is tapped into a ladle, which is carried by a ladle-car into position to discharge into the double-mouthed trough. As each mold passes it is filled with molten iron. The endless chain of molds as it moves along descends under a tank of water, where the molten metal cools and hardens. The cooling is continued by sprays of water as the molds ascend from the water and move on toward the tail sheaves, where the pigs are discharged onto cars. The casting machine of one of the most recent American blast-furnace plants has a chain travel of 20 feet per minute, the molds are spaced 1 foot apart on the chains, and the machine delivers 20 pigs per minute averaging in weight 110 pounds each.
In the preceding description of the blast-furnace process of producing cast iron the attempt has been made to avoid confusion by subordinating the accessory details of furnace equipment and operation to the main narrative. Some of these matters deserve further mention. The essential accessories to the blast-furnace in the nature of a working plant are the blowing engine, the hot-blast stove, and the material hoist. The older and less-used form of blowing engine is the beam engine, the steam-cylinder being connected to one end of the beam, and the air or blowing cylinder to the other. The present form of blowing engine is either a vertical or a horizontal direct-acting engine, the former being the more common type. Very often the steam end of the engine is compound. Until very recently steam blowing engines have been used almost exclusively, but in Europe considerable progress has been made within the last few years in the adoption of gas-engines (q.v.) operated by blast-furnace gas. In America a separate engine is generally provided for each furnace, but in Europe one engine commonly supplies air to two or more furnaces. The blast pressure provided by blowing engines in actual working varies from 5 pounds to 14 pounds per square inch, American iron-makers favoring the higher pressure.
The first form of hot-blast stoves were all heated by solid fuel. About 1860 E. A. Cowper. an Englishman, applied the regenerative method of Siemens to heating the blast with waste furnace gases. The Cowper stove, or modifications of it, are now extensively used. Two stoves are required for each furnace. Briefly described, each stove consists of an outer shell of steel plates lined with fire-brick to form a vertical cylinder with a dome-shaped top. A circular flue extends from the bottom to the top of the stove, and the remainder of the space is filled with special-shaped fire-bricks which form a honeycombed or cellular filling. Hot gases from the furnace are discharged into the bottom of the vertical flue, where they meet the air-inlet and combination takes place. The burning gases pass up the flue and thence down through the cellular filling to an outlet leading into the chimney. In passing through the cellular filling the gases heat the brick to a red heat. When this is accomplished the gases are shut off by means of suitable valves, and by opening other valves the cold blast from the blowing engine is passed through the stove in the direction opposite to that taken by the heating gases, and thence through the tuyeres into the furnace, being highly heated in the passage. Meanwhile the gases diverted from the first stove are being passed through the second stove and are heating it; that is, one stove is absorbing heat while the other is heating the blast. Sometimes a third stove is supplied to be used in case of accident to one of the others. The Whitwell stove is another popular form. It is a cylindrical sheet-steel, fire-brick lined structure like the Cowper stove; but the interior construction consists of a series of vertical fire-brick passages through which the hot gases and blast-air pass alternately up and down, but in opposite directions. The operation of the Whitwell stoves is similar to that of the Cowper, one receiving heat from the furnace gases while the other is heating the blast. Several combinations and modifications of the Cowper and Whitwell stoves are in use.
The material hoist is a necessary adjunct to the modern blast-furnace, where hundreds of tons of coke, ore, and limestone have to be raised daily 100 feet or more to the charging hopper. These hoists are operated by hydraulic, pneumatic, or steam power, and in general consist of an elevator cage or corresponding apparatus on which the material cars may be raised and lowered. In the furnace illustrated the cars are hauled up and down an inclined plane. These hoists are arranged to work as nearly automatically as possible. Among the other appliances necessary to the operation of a modern blast-furnace are pumping engines for supplying cool water to the hearth-jacket and to the cooling rings and for other purposes; dust-catchers for separating the dust from the furnace gases previous to their utilization in the hot-blast stove, or for other purposes; the tuyeres: apparatus for tapping the furnace; ladles of various kinds, etc.
A few words may now be said regarding the fuel and fluxes used in blast-furnace smelting. As has been said already, charcoal was the fuel originally employed. At present but few charcoal furnaces are in operation, but the iron produced from them is of the best quality, and is much used for special purposes. Coke is the fuel most extensively used, and its manufacture is fully described in the article Coke. Next to coke, coal is the most commonly used fuel. Attempts have been made in a few instances to use gaseous fuel, but they have met with little success. The flux universally used in smelting iron is limestone. The purpose and action of this flux has been explained briefly in describing the chemical reactions which take place in the blast-furnace.
Properties of Cast Iron. The product of the blast-furnace, as already said, is cast iron. As this is the preliminary form of practically all iron for making both wrought iron and steel, its properties require a brief description. Cast iron consists of metallic iron combined with at least 1½ per cent. of carbon and smaller percentages of silicon, sulphur, phosphorus, and manganese; the elements other than metallic iron being about 7 per cent. of the whole, though this percentage may vary considerably. Cast iron melts at about 1200° C., and when cold is hard and brittle. It is not malleable or ductile, and cannot be tempered. Of the several elements named, carbon and silicon are of value in steel-making, which is the chief purpose for which cast iron is produced at the present time; but sulphur and phosphorus are generally highly objectionable. In using the term cast iron here, the chemical nomenclature has been retained. The foundryman generally gives the name pig iron to the direct product of the blast-furnace, and the term cast iron to the form which pig iron assumes after remelting. For commercial purposes pig iron is graded into several classes, according to the appearance of the fracture. This is an unscientific and unsatisfactory method of grading, but it is the one almost always adopted. The different grades are given different names, and these names vary in character and significance in different countries. In the United States the more common grades are: No. 1 Foundry, No. 2 Foundry, No. 3 Foundry, No. 1 Soft, No. 2 Soft, Silver Gray, Gray Forge, Mottled, and White. These grades vary in chemical composition, and it is being urged by many prominent metallurgists that chemical composition be substituted for the appearance of the fracture in distinguishing between different grades of iron.

|
A MODERN BLAST FURNACE
Blast Furnace of Colorado Fuel and Iron Company at Pueblo, Colorado. Capacity, 600 Tons.
Wrought Iron. The iron made by the ancients, and, indeed, by all peoples up to 1350, when cast iron was first produced in the German blast-furnaces, was wrought iron, and it was produced from the ore in a single operation. Today wrought iron is nearly always obtained by treating pig or cast iron made in the blast-furnace, and the process is said to be ‘indirect,’ as compared with the ‘direct’ process of the ancients. The direct process is still employed by all savage races who make iron, and is also in use where the character of the ore, the fuel, or other conditions make the adoption of the blast-furnace impracticable. A few forms of the direct process will be described here. The ancients employed small hearths, the fuel used being charcoal and the necessary draught being obtained either by means of rude bellows or by arranging the hearth at the top of a gully or channel in such a manner as to take advantage of the prevailing wind. Such hearths are now used in Africa and some parts of India by the natives. A modification of the hearth process once extensively used in Southern Europe, but now extinct, was the Catalan process, the name being derived from the Province of Catalonia, in Spain, where the process was probably first employed. This process is chiefly notable because of the form of water blower used, which was called a trompe. See Blowing Machines.
A more important hearth process was the American bloomery process, much resembling the Catalan process, but showing an advance in the use of more modern blowing machines with hot blast, and in the provision of means for cooling the furnace walls by water. The three processes described were hearth processes. A more common form of the direct process consists in the use of blast-furnaces. These furnaces are insignificant in size, as compared with modern cast-iron blast-furnaces: the smaller ones are from 4½ to 6 feet in height, while the largest seldom exceed 10 feet in height. These furnaces are extensively used by the natives of India, who produce an excellent quality of iron in them. The two modern forms of the direct process are the retort process and the reverberatory furnace process. In the retort process, of which there are several forms, the ore, reduced to a line division, is placed in the heating chamber with charcoal or other carbonaceous matter, and is heated either by the external fire or by means of gaseous fuel, the products of combination of which are made to pass through the charge. In the reverberatory-furnace process fine ore and coke are reduced in a reverberatory furnace by gaseous fuel. In all of these processes thus far mentioned the direct product of the furnace is a spongy mass of metallic iron and slag, which has to be squeezed or hammered to remove the bulk of the cinder.
The preceding brief review of the direct process of producing wrought iron may be summed up by saying that at present there is no direct process known which is capable of competing for a lengthened period and on a broad scale with the indirect process. The manufacture of wrought iron from cast iron by this process is accomplished by purification. This further purification is always carried out by means of oxidation, though the details of the process vary according to whether the necessary oxygen is supplied chiefly from the atmosphere or from other materials added for the purpose, and also as to whether the iron to be purified is heated in a separate furnace or chamber from that in which the fuel is burned, or in direct contact with the fuel. The furnaces used may be divided into two classes—(1) hearths and (2) reverberatory furnaces. In hearths the fuel is burned in direct contact with the iron, and the chief source of oxygen is the atmosphere; in the reverberatory furnace the oxygen is obtained from special oxidizing materials added for the purpose, and the fuel is burned in a chamber separate from, but communicating with, the chamber in which the charge is placed. With this outline of the methods of producing wrought iron from cast iron, attention will be turned to the process which is chiefly employed.
The chief impurities to be removed from cast iron to make it into wrought iron are silicon, manganese, phosphorus, and carbon. To remove these by the puddling process, a special puddling furnace is employed. These furnaces are of various forms, but the ordinary form is a single-bedded reverberatory furnace. Briefly described, such a furnace consists of a combination chamber of oblong shape. At the front of this chamber are the grate-bar, to the rear of which rises a vertical wall, and back of this, at a higher level than the grate-bars, is a shallow receptacle for the charge. A common roof covers the two chambers. This roof is horizontal over the grate-bars, but curves downward as it extends backward until at the flue-entrance it descends below the level of the charge. There are two doors at the side, one for feeding the fire and the other to give access to the charge. Structurally the furnace consists of cast-iron plates and firebrick. In operation the combustion of the fuel takes place on the grate-bars, and the hot gases and flames rise to the roof and are beaten back or reverberated upon the charge as they move toward the flue. Generally two men work at a furnace. The puddling process consists of four stages. Previous to describing these stages it will be necessary to state briefly the manner of preparing the furnace for work. The basin for the charge is made of cast-iron plates. These are covered with a layer of oxidizing material a few inches deep, and the fire is then started and urged until the heat is intense enough to partly fuse this material and the fragments cohere. The sides of the basin are banked up with similar material. Slag is then shoveled in, and the pig iron to be treated is placed in this couch of slag. The lining of the basin lasts for many successive charges, with occasional repairs as wear occurs.
The furnace having been charged, the door is closed, and rendered as nearly as possible air-tight by banking with cinder. Heating is continued until the top of the pig iron is red hot, when it is turned by opening the door, which is afterwards closed until the iron melts, the workmen stirring up the mass with a rod at intervals to hasten the process. This completes the melting-down stage. One of the workmen next introduces a hooked bar and vigorously stirs the molten mass until its appearance to his skilled eye indicates that the silicon has been expelled. This completes the second or clearing stage. The next process is to reduce the temperature, and vigorously continue stirring until the metal and cinder become thoroughly mingled, part of the slag flows off, and the metallic iron and residue of slag form a porous cake in the melting basin. This completes the third stage, known as the boil. The final stage is to break up the porous cake, and form the separate parts into balls by manipulating them with a bar. These balls are taken from the furnace and hammered or squeezed to press out the slag, when the iron is ready to be manufactured. During these various stages of the puddling process the impurities of the cast iron (silicon, manganese, phosphorus, and carbon) are removed. As previously stated, various forms of puddling furnace are used, and other variations in apparatus and methods employed; but the general process is always essentially as described.
The crude puddle ball which is drawn from the furnace is composed of innumerable globules of nearly pure iron, the interstices between which are filled with slag. Much of this slag is removed in squeezing, and each subsequent working removes a further quantity, but it is never all removed. The piece of iron made in the first rolling of the puddle ball is a rough, crude product known as muck bar. To make merchant iron several of these muck bars are bundled together into ‘piles,’ so as to give a bloom of proper sectional area, and this after being heated to a welding heat is rolled into the desired shape. See Rolling-Mill.
Steel. As has been noted, steel was manufactured at a very early date in the history of civilization. Prior to 1856, however, it was produced in comparatively small amounts, and its use was restricted to the production of cutlery and tools. In 1856 Henry Bessemer made known the process for making steel in large quantities which revolutionized the iron trade of the world. This was followed by the inventions of Siemens, Martin, and Thomas, which gave a further impetus to steel manufacture, and widely extended the use of that material. At present steel is made by the cementation process, by the crucible process, by the acid Bessemer process, by the basic Bessemer process, by the acid open-hearth process, and by the basic open-hearth process. In the following paragraphs each of these processes will be described in outline.
Cement steel is made by placing a bar of soft, pure wrought iron in fine charcoal and exposing it to yellow heat. By a slow process called cementation the carbon penetrates the metal at the rate of about one-eighth inch every twenty- four hours. The process of cementation is carried on in large retorts which handle many tons of bars at one time, so that it will always happen that some parts of the furnace arrive at full heat much sooner than others, and remain longer at that temperature. The consequence is that it is necessary to break all the bars and grade the pieces by fracture according to their degree of carburization. Steel made in this way is commonly known as blister steel. Its use is limited by the fact that it always contains seams or pits of slag, which are present in the wrought iron. To avoid this trouble cement steel may be melted in a crucible out of contact with the air, and, being thus free from the slag, can be cast into ingots and hammered or rolled into any desired shape. This double process is expensive, and a cheaper and more common method of making crucible steel is to place powdered charcoal and crude bar iron in the crucible, the iron absorbing the carbon very rapidly in the molten state. This practice is almost universal in America. Sometimes pig iron and wrought iron are melted together, and in Sweden crucible steel is produced from pig iron and iron ore. Both blister steel and crucible steel belong to the general class known as high-carbon steel. Such steel can be made regularly in open-hearth furnaces; but so far this method, though cheaper, has not replaced the older methods. Blister and crucible steel are chiefly used for high-class edged tools, springs, etc.
Bessemer Process. The most common steel-making process is the Bessemer process, which may be subdivided into the acid Bessemer and the basic Bessemer process. The apparatus used is the same for both the acid and the basic process, and the general process is the same up to a certain point. The chemical reactions differ substantially, however. Briefly described, the Bessemer process consists in charging molten pig iron into a vessel called a converter, forcing a blast of air through it until the silicon, manganese, and carbon are burned out, and restoring a definite portion of manganese and carbon by adding a recarburizing material. This is the process in skeleton; it divides itself for the purpose of a detailed consideration into the following divisions: Apparatus and mechanical manipulations and chemical reactions.
The central feature of the plant for making Bessemer steel is the converter. This is a pear-shaped or jug-shaped vessel of steel, lined with a refractory material. Fig. 2 shows a modern American Bessemer converter in cross-section. The vessel is mounted on a horizontal axis, consisting of two hollow gudgeons, through which the air-blast enters the bottom of the converter. An automatic valve shuts off the air when the converter is turned on its side, and admits it when the converter is upright. The blast is furnished by a blowing engine which keeps the pressure at from 25 pounds to 30 pounds per square inch. The converter lining is about one foot thick, and consists of a siliceous composition or stone in the acid process, and of dolomite or limestone in the basic process. The converter is so equipped that it can be rotated from a vertical to a horizontal position and back in either direction. In operation the molten pig iron is charged into the converter when it lies horizontal. When the molten metal is taken directly from the blast-furnace it is usually brought to the converter in ladles; but in case the iron is melted in cupola furnaces these are to placed that they discharge directly into the converter. As soon as the charge has been run into the converter it is turned into an upright position, the operation automatically turning on the blast. The blowing continues from seven to twelve minutes, and then the converter is turned upon its side and the recarburizing material in molten form is added. The charge is then ready for casting. This operation consists first in drawing the contents of the converter into a ladle, which is swung into position under the nose of the converter by a crane, the converter being tipped so as to empty. Sometimes the molds are set in a row around the perimeter of a circular pit, and the ladle is swung around in a circle by the crane to fill one mold after another; but more often, in American practice, the molds are mounted on little platform cars which are hauled past the ladle and filled one at a time, the same cars taking the filled molds to the rolling-mill, where they are stripped from the ingots. Practically all the mechanical operations, like the tilting of the converter and ladles, are performed by power.
The chemical reactions which take place in the converter differ according to whether the converter is acid- or basic- lined. In both cases the object is to burn away the silicon and carbon of the pig iron, and then to add carbon in the proper proportions to make steel. The reason for this seemingly inconsistent practice of first burning away the carbon and then adding the same material is as follows: Pig iron contains varying quantities of carbon, and to burn away just enough would necessitate a different length of the blowing for each charge of iron, thus introducing complications difficult to handle. It was Bessemer's original plan to do this, however, and it delayed the general introduction of the process until the remedy was found by Mushet. This consisted in burning out the impurities and then adding a definite amount of carbon and manganese in the form of molten spiegeleisen or ferromanganese.
When air is blown through molten pig iron in a Bessemer converter, the first element affected is the silicon, and when the silicon is eliminated the carbon begins to burn and continues until there is only about 0.5 per cent. Up to the point where the carbon content has been reduced to 0.5 per cent., the reactions of the acid and of the basic processes are the same, but at this point the similarity ceases, for here the acid process ends, while the basic process begins its characteristic work of eliminating the phosphorus and sulphur. For practical purposes it may be assumed that neither the phosphorus nor sulphur contents of the original pig iron have altered at the time that the carbon content has reached 0.5 per cent. From that time on the phosphorus seizes the oxygen in the same way as the silicon and carbon had done before, the phosphoric acid immediately uniting with the lime which in the basic process is added to the metal at the beginning of the blow. The basic lining of the furnace is employed so that the lime may do its work without being affected by the lining material; were an acid lining used the silica would combine with the lime, thus rendering much of it incapable of doing useful work on the metal. The decarbonization, dephosphorization, etc., effected by the blowing process require to be supplemented by a further process before the final product of the converter is steel. This process is known as recarburization, and consists in adding carbon and manganese to the molten metal by the use of spiegeleisen or ferromanganese; the manganese promotes the removal of the sulphur with the slag. The amount of these materials to be added varies with the character of steel it is required to produce, and also with the process.
As has been stated, the process of steel-making which has just been described was the invention of Henry Bessemer, an Englishman. It developed from his efforts to produce a stronger metal than cast iron for the manufacture of ordnance. In 1854 James Nasmyth had patented a process for oxidizing the impurities of molten cast iron by introducing steam below the surface of the metal. Bessemer substituted air for steam, and patented the process in 1855. In his first experiments, Bessemer devoted his attention to the production of malleable iron, and such was his success that in 1856 he announced his work to the public. At this time, however, the process was far from perfect. Bessemer soon discovered that, while his process would remove silicon, manganese, and carbon from cast iron, it would not, when conducted in an acid-lined converter, remove phosphorus and sulphur. After some attempts to accomplish the removal of the last two elements, which met with poor success, Bessemer was compelled to revert to the use of iron so low in phosphorus and sulphur that it would make steel without any further diminution of these elements. Thus arose a classification of Bessemer pig, which still exists (see previous section on Cast Iron), and which means simply pig iron so low in phosphorus and sulphur that it may be made into steel without removal of these elements. The question of phosphorus being thus satisfactorily settled, Bessemer was confronted with the difficulty of so regulating the period of blowing that carbon should not be eliminated below the amount required in steel. As stated, this problem was solved by burning out practically all of the carbon and then adding a definite amount in the form of a recarburizer. In the same way, the necessary quantity of manganese was supplied. The success of the Bessemer process was not established commercially until 1860; from that date it has grown by leaps and bounds, until to-day it is perhaps the most important of the steel-making processes. Bessemer reaped fame and wealth from his invention, of which it has been truly remarked that it was of far more importance to the world than all the gold of California and Australia.
The open-hearth process of steel-making consists in making pig iron mixed with a greater or less quantity of wrought iron, steel, or similar iron products by exposure to the direct action of flame in a regenerative gas-furnace, and converting the resultant bath into fluid steel. Like the Bessemer process, the open-hearth process is divided into an acid process and a basic process; in the first the hearth is lined with sand and the slag is siliceous, and in the second the hearth is lined with a basic material and the slag is basic. Like the Bessemer process, also, the consideration of the open-hearth process may be divided into a discussion, first of the plant and mechanical operations, and then of the chemical reactions.
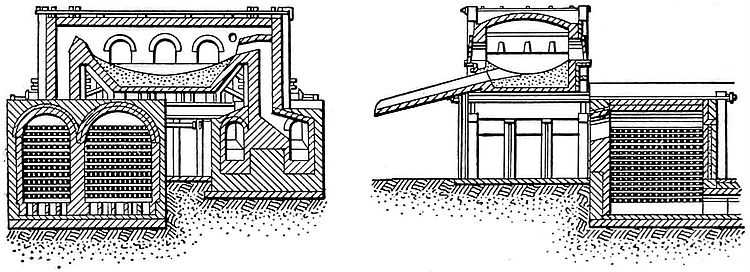
The central figure of an open-hearth steel plant is the melting-furnace. This is always a regenerative furnace, for in no other construction is it practicable to obtain the necessary temperature, but its construction varies greatly in other respects. Fig. 3, showing a furnace designed for the Illinois Steel Company, of Chicago, may be used to explain the general features of construction and operation of an open-hearth steel furnace. In this drawing the checker-work shows the regenerating chambers, and the saucer-shaped structure above is the hearth. The two regenerating chambers are separate from each other, but either may be connected with the hearth and with the smokestack. Each chamber is nearly filled with a sort of cob-house work of brick. In operation, one chamber is heated so that the brick filling is white hot, and then a current of air and gas is admitted at the bottom. As this combustible mixture passes up through the heated brickwork, it is heated so that when it arrives at a point over the hearth it is burning fiercely. A portion of the heat of this combustion is given up to the charge on the hearth. From the hearth the hot gases pass down through the brickwork of the second chamber and serve to heat it, after which they escape at the bottom into the smokestack flue. As soon as the brickwork of the first chamber has become cooled below a certain point, the current of gases is reversed, so that they enter at the bottom of the second chamber and pass out of the bottom of the first chamber after being heated and ignited by the hot brickwork through which they have passed, and after passing over the hearth giving up their waste heat to the brickwork of the first chamber. By repetitions of this process of reversing the current of gases, the regenerative process is continuous, and a steady, intense heat is maintained on the hearth.
The hearth is usually a fixed structure, as shown by Fig. 3, but in some American works it is so constructed that it can be tilted like a Bessemer converter to receive its charge and to discharge its molten contents. This form of hearth is claimed to have several material advantages over the fixed hearth in case of operation and in producing steel from certain materials for certain special purposes. Whether fixed or tilting, the construction of the hearth consists of a steel and iron plate shell lined with some refractory material. In hearths for the acid process, the hearth-lining proper consists first of a layer of sand fused into a solid mass by heat. In hearths for the basic process the lining consists of a plastic compound made by roasting and grinding dolomite limestone and mixing the powder with tar. The accessories to the hearth consist of charging and tapping appliances, ladles and cranes for handling them, casting molds, etc.

|
OPEN-HEARTH FURNACE
Forty-Ton Open-Hearth Furnace at the Homestead Steel Works of the Carnegie Steel Company.
In respect to their chemistry, the acid and the basic open-hearth process can best be considered separately. Referring first to the acid process, the proportions of the constituents of the charge vary in different places. Sometimes pig iron alone is used, but more generally pig iron and scrap wrought iron and steel are mixed. Whatever the mixture may be, it is necessary that its contents of phosphorus and sulphur be known, since these are not eliminated in the process. Broadly considered, the chemical reactions may be divided into three operations: first, the reactions during melting; second, the reactions after melting; and third, the reactions during recarburization. During melting the silicon and manganese are reduced by oxidization to a minute fraction of their original amounts and about one-half of the carbon eliminated. After melting, the remaining silicon, manganese, and carbon are eliminated by keeping the molten metal at a high heat and adding iron ore in successive small doses, thus forming silica and oxide of manganese, which go into the slag, and carbonic oxide, which escapes with the flame. To determine when this process has proceeded far enough, samples of the molten metal are taken at intervals, cast into iron bars and broken; the carbon content is estimated by the appearance of the fracture, an expert being thus able to determine its amount with much accuracy. When the desired point of carbon content has been reached, as determined by the test, the recarburizer is added in a solid state. This recarburizer is ferromanganese with a very large excess of manganese. In the basic process the problem is the melting and decarburization of the charge, as in the acid process just described, with the additional duty of removing a reasonable quantity of the phosphorus. This is accomplished by adding lime to the charge, which takes up the phosphorus and confines it in the slag. The basic lining in the furnace is necessary to leave the lime free to perform useful work, which would not be the case were an acid lining used which would take up a portion of the lime.
Recarburization is accomplished in much the same way as in the acid process. Summarized, the chemical problem of the open-hearth process is to eliminate from the crude iron of the charge all the silicon, manganese, carbon, phosphorus, and sulphur in excess of the amounts required for steel. The problem is practically the same in the Bessemer process, but the method of its solution is different. In the Bessemer process the metal is always blown until nearly all the carbon is eliminated, since it has been found impracticable to stop the operation at any intermediate point. All the carbon content of Bessemer steel has, therefore, to be supplied by the recarburizer, and absolutely perfect homogeneity of product can be secured only by absolutely perfect mixing of the molten metal and the recarburizer. This perfect mixing increases in difficulty as the amount of carbon required in the steel increases. In the open-hearth process the elimination of the carbon can be stopped at any desired point, so that very little carbon is added in the recarburizer, and the necessity of thorough mixing is less imperative. As a result it is generally considered that high carbon steel or hard steel can be produced with a more uniform quality by the open hearth than by the Bessemer process.
An important modification of the open hearth which has been described, and which is intermittent in operation, is the Talbot continuous open-hearth process, now in use in some American steel-works and being installed in several others here and abroad. Mr. Talbot's process consists essentially in working the furnace continuously by tapping off a portion of the molten charge at short intervals, immediately charging an equivalent of pig iron, and again tapping. Several advantages in increased output, economy of maintenance, wider range, etc., are claimed for the process.
The development of the open-hearth process of steel-making was the outgrowth of numerous attempts by inventors to repeat the success of Bessemer. Not much success was realized in these efforts, however, until 1862, when W. Siemens, a German, applied the regenerative furnace, which he had invented in 1857, to the manufacture of steel. It was not until 1868, when Siemens succeeded in making steel from old iron rails, that the success of the process was fully demonstrated. Meanwhile, P. and E. Martin, of Sireul, France, had succeeded in making steel from a mixture of pig iron and scrap in a Siemens furnace. Thus originated the Siemens-Martin process or open-hearth process of steel-making. At present, this process ranks second in importance only to the Bessemer process. The Bessemer process is practically without rival for the production of steel rails, but the open-hearth process leads in the production of structural steel, ship's plates, and steel for castings.
At first both the Bessemer and the open-hearth process were employed only with acid-lined furnaces, the basic process being a subsequent development. The practical invention of the basic process was due to Sidney G. Thomas and P. C. Gilchrist, and was first made public in 1878. The essential idea of the invention consisted in the substitution of a basic lining instead of the acid lining previously used in both the Bessemer and open-hearth processes, and the addition of a quantity of quicklime during the process so as to combine with the silicon and phosphorus, and thus to save the lining as much as possible. The success of the invention was not demonstrated until 1879, but since that time the process has developed rapidly. By this invention the enormous deposits of iron ores high in phosphorus, which had previously been excluded from use in the two great steel-making processes of the world, were rendered available to the steelmakers.
Physical Properties of Iron and Steel. The physical properties of iron and steel which are chiefly useful to the engineer and manufacturer are strength, hardness, weldability, ductility, malleability, elasticity, and homogeneity. With the exception of weldability, all of these properties are exhibited to some extent by every piece of iron or steel. The relative degrees in which these different properties exist in different kinds of iron and steel vary greatly, however. This is a familiar fact to all, and does not need proof. The variation in these properties in different kinds of iron and steel is due partly to variation in the relative amounts of the contained chemical elements, partly to the physical structure, and partly to the method and amount of working to which the metal has been subjected to attain its final useful form. Commercially, iron and steel may be divided into the following general classes: Wrought iron, soft steel, medium steel, hard steel, cast steel, hard cast steel, cast iron, malleable cast iron. The chief physical properties of each of these classes of iron and steel will be referred to after a brief statement of the destructive effects produced by the different chemical elements upon the physical properties of iron and steel generally. The effects of carbon on iron and steel are more pronounced and useful than those of any other known chemical element; it constitutes about 4 per cent. of cast iron, and from ½ to 1½ per cent. of steel, and is nearly entirely absent from wrought iron. The effects of increasing the carbon element in iron or steel are to increase the hardness, strength, and fusibility, and to decrease the ductility, malleability, and weldability. The effect of increasing the silicon element of iron and steel is to increase the strength and soundness of steel and the fluidity of cast iron, and to reduce the ductility of steel. Manganese has the effect of counteracting the injurious effects of sulphur, phosphorus, and some other impurities, and increases hardness, fluidity, elasticity, and strength. Sulphur and phosphorus are nearly unmixed evils in iron and steel, and every effort is made to remove them from these metals.
The effect of none of these elements upon the metal is independent, but is influenced by the presence of one or more of the others. These interactions are too complicated to be discussed outside of special technical treatises. The effect of treatment during working upon the physical properties of iron and steel are numerous, and are referred to in the articles on Annealing; Forge, Forging; Founding; Rolling-Mill; Wire.
Turning now to the physical properties of the several classes of iron and steel mentioned above, it may be noted, first, that strength may be subdivided into strength against rupture by direct pull, or tensile strength; strength against rupture by compression, or compressive strength; strength against rupture by bending, or flexural strength; and strength against shear, or shearing strength. (For the methods of measuring these various forms of strength, see Strength of Materials.) Hardness is the capacity to resist indentation; weldability is the property which permits two or more separate fragments to be welded together; ductility is the property which renders the metal capable of being drawn out into rods or wire; malleability is the property which permits the metal to be hammered or pressed into different shapes; elasticity is the property which gives the metal power to return to its original form after distortion; and homogeneity is the property which secures uniformity of structure and mass.
Cast iron cannot be welded like wrought iron, and its malleability and ductility are practically nil; its tensile strength is from 15,000 pounds to 35,000 pounds per square inch; its compressive strength is from 60,000 pounds to 200,000 pounds per square inch; its flexural strength is from one-half to two-thirds its tensile strength; its elasticity is small. Wrought iron is malleable, can be forged and welded, and has a high capacity to withstand the action of shocks; it cannot be tempered, and melts only at the highest temperatures. Its tensile and compressive strengths are closely equal, and range from 50,000 pounds to 60,000 pounds per square inch; it is tough and ductile. The physical properties of steel depend upon the chemical composition and method of manufacture, and they vary so greatly, both relatively and absolutely, that no effort will be made to define them here. The mild and soft structural steels for bridges and buildings have tensile strengths of from 60,000 pounds to 70,000 pounds per square inch, with a limit of elasticity of from 30,000 pounds to 40,000 pounds per square inch. The hard steels have a much greater strength. The compressive strength of steel is always greater than the tensile strength.
Uses of Iron and Steel. The uses to which iron and steel are put are so familiar that only brief mention need be made of them. Cast iron is used chiefly in founding or iron-casting, the varieties and purposes of such castings being almost innumerable. Cast steel is used for many of the same purposes as cast iron, but more particularly for those purposes where castings of great strength are required. Wrought iron is forged into various shapes for special purposes, and is rolled into bars, plates, beams, rails, and structural shapes. Extra hard steels are used for tools, hard steel for piston-rods and other parts of machines, medium steel for rails and guns, and the mild and soft steels for beams and structural purposes.
Statistics of Production. The following table, rearranged from statistics published in The Mineral Industry (New York) for 1901, shows the production of iron and steel in the principal countries of the world for the twelve months of 1900:
| COUNTRY | Pig Iron, metric tons |
Steel, metric tons |
| Austria-Hungary | [1]1,350,000 | [1]676,000 |
| Belgium | 1,018,507 | 654,827 |
| Canada | 87,612 | |
| France | 2,699,424 | 1,624,048 |
| Germany | 8,351,742 | 6,645,869 |
| Italy | [1]20,000 | [1]58,000 |
| Russia | [1]2,850,000 | [1]1,500,000 |
| Spain | 294,118 | 150,634 |
| Sweden | 520,000 | 291,900 |
| United Kingdom | 9,052,107 | 4,800,000 |
| United States | 14,099,870 | 10,382,069 |
| All other countries | [1]625,000 | [1]400,000 |
| Totals | 40,968,980 | 27,182,347 |
As will be noted, the three great iron and steel producing countries of the world are Germany, the United Kingdom, and the United States.
Taking up the figures for the United States in somewhat more detail, we have the following, showing the relative output of the different classes of steel and iron:
| Pig Iron | ||
| CLASS | Long tons | Per cent. |
| Foundry and forge | 4,517,437 | 32.8 |
| Bessemer pig | 7,943,452 | 57.6 |
| Basic pig | 1,072,376 | 7.8 |
| Spiegeleisen and ferromanganese | 255,977 | 1.8 |
| Total | 13,789,242 | 100.0 |
| Steel | |
| CLASS | Long tons |
| Bessemer | 6,684,770 |
| Open-hearth | 3,402,552 |
| Crucible and miscellaneous | 131,250 |
| Total | 10,218,572 |
The principal pig-iron producing States of the United States in 1901 were: Alabama, 1,225,212 tons; Illinois, 1,596,850 tons; Ohio, 3,926,425 tons; and Pennsylvania, 7,343,257 tons.
Bibliography. For a comprehensive discussion of the metallurgy of iron and steel, consult: Howe, Metallurgy of Steel (New York, 1890); Campbell, The Manufacture and Properties of Structural Steel (New York, 1896); and Turner, The Metallurgy of Iron (London, 1895). For details of the manufacture of iron and steel into special forms, see Founding; Forge, Forging; Rolling-Mill; Wire.