deduced the relative weight of the oxygen atom to be 6⋅5; from marsh gas and olefiant gas he deduced carbon=5, there being one atom of carbon and two of hydrogen in the former and one atom of hydrogen to one of carbon in the latter; nitrogen had an equivalent of 5, and so on.[1]
The value of Dalton’s generalizations can hardly be overestimated, notwithstanding the fact that in several cases they needed correction. The first step in this direction was effected by the co-ordination of Gay Lussac’s observations on the combining volumes of gases. He discovered that gases always combined in volumes having simple ratios, and that the volume of the product had a simple ratio to the volumes of the reacting gases. For example, one volume of oxygen combined with two of hydrogen to form two volumes of steam, three volumes of hydrogen combined with one of nitrogen to give two volumes of ammonia, one volume of hydrogen combined with one of chlorine to give two volumes of hydrochloric acid. An immediate inference was that the Daltonian “atom” must have parts which enter into combination with parts of other atoms; in other words, there must exist two orders of particles, viz. (1) particles derived by limiting mechanical subdivision, the modern molecule, and (2) particles derived from the first class by chemical subdivision, i.e. particles which are incapable of existing alone, but may exist in combination. Additional evidence as to the structure of the molecule was discussed by Avogadro in 1811, and by Ampère in 1814. From the gas-laws of Boyle and J. A. C. Charles—viz. equal changes in temperature and pressure occasion equal changes in equal volumes of all gases and vapours—Avogadro deduced the law:—Under the same conditions of temperature and pressure, equal volumes of gases contain equal numbers of molecules; and he showed that the relative weights of the molecules are determined as the ratios of the weights of equal volumes, or densities. He established the existence of molecules and atoms as we have defined above, and stated that the number of atoms in the molecule is generally 2, but may be 4, 8, &c. We cannot tell whether his choice of the powers of 2 is accident or design.
Notwithstanding Avogadro’s perspicuous investigation, and a similar exposition of the atom and molecule by A. M. Ampère, the views therein expressed were ignored both by molecular weights, attention was concentrated their own and the succeeding generation. In place of the relative molecular weights, attention was concentrated on relative atomic or equivalent weights. This may be due Berzelius.in some measure to the small number of gaseous and easily volatile substances then known, to the attention which the study of the organic compounds received, and especially to the energetic investigations of J. J. Berzelius, who, fired with enthusiasm by the original theory of Dalton and the law of multiple proportions, determined the equivalents of combining ratios of many elements in an enormous number of compounds.[2] He prosecuted his labours in this field for thirty years; as proof of his industry it may be mentioned that as early as 1818 he had determined the combining ratios of about two thousand simple and compound substances.
We may here notice the important chemical symbolism or notation introduced by Berzelius, which greatly contributed to the definite and convenient representation of chemical composition and the tracing of chemical reactions. The denotation of elements by symbols had been practised by the alchemists, and it is interesting to note that the Chemical notation.symbols allotted to the well-known elements are identical with the astrological symbols of the sun and the other members of the solar system. Gold, the most perfect metal, had the symbol of the Sun, ☉; silver, the semiperfect metal, had the symbol of the Moon, ☽; copper, iron and antimony, the imperfect metals of the gold class, had the symbols of Venus ♀, Mars ♂, and the Earth ♁; tin and lead, the imperfect metals of the silver class, had the symbols of Jupiter ♃, and Saturn ♄; while mercury, the imperfect metal of both the gold and silver class, had the symbol of the planet, ☿. Torbern Olof Bergman used an elaborate system in his Opuscula physica et chemica (1783); the elements received symbols composed of circles, arcs of circles, and lines, while certain class symbols, such as ![]() for metals,
for metals, ![]() for acids,
for acids,
![]() for alkalies,
for alkalies, ![]() for salts,
for salts, ![]() for calces, &c., were used. Compounds were represented by copulating simpler symbols, e.g. mercury calx was
for calces, &c., were used. Compounds were represented by copulating simpler symbols, e.g. mercury calx was ![]()
![]() .[3] Bergman’s symbolism was obviously cumbrous, and the system used in 1782 by Lavoisier was equally abstruse, since the forms gave no clue as to composition; for instance water, oxygen, and nitric acid were
.[3] Bergman’s symbolism was obviously cumbrous, and the system used in 1782 by Lavoisier was equally abstruse, since the forms gave no clue as to composition; for instance water, oxygen, and nitric acid were ![]() ,
, ![]() , and
, and ![]() .
.
A partial clarification was suggested in 1787 by J. H. Hassenfratz and Adet, who assigned to each element a symbol, and to each compound a sign which should record the elements present and their relative quantities. Straight lines and semicircles were utilized for the non-metallic elements, carbon, nitrogen, phosphorus and sulphur (the “simple acidifiable bases” of Lavoisier), and circles enclosing the initial letters of their names for the metals. The “compound acidifiable bases,” i.e. the hypothetical radicals of acids, were denoted by squares enclosing the initial letter of the base; an alkali was denoted by a triangle, and the particular alkali by inserting the initial letter. Compounds were denoted by joining the symbols of the components, and by varying the manner of joining compounds of the same elements were distinguished. The symbol ∨ was used to denote a liquid, and a vertical line to denote a gas. As an example of the complexity of this system we may note the five oxides of nitrogen, which were symbolized as
the first three representing the gaseous oxides, and the last two the liquid oxides.
A great advance was made by Dalton, who, besides introducing simpler symbols, regarded the symbol as representing not only the element or compound but also one atom of that element or compound; in other words, his symbol denoted equivalent weights.[4] This system, which permitted the correct representation of molecular composition, was adopted by Berzelius in 1814, who, having replaced the geometric signs of Dalton by the initial letter (or letters) of the Latin names of the elements, represented a compound by placing a plus sign between the symbols of its components, and the number of atoms of each component (except in the case of only one atom) by placing Arabic numerals before the symbols; for example, copper oxide was Cu+O, sulphur trioxide S+3O. If two compounds combined, the + signs of the free compounds were discarded, and the number of atoms denoted by an Arabic index placed after the elements, and from these modified symbols the symbol of the new compound was derived in the same manner as simple compounds were built up from their elements. Thus copper sulphate was CuO+SO3, potassium sulphate 2SO3+PoO2 (the symbol Po for potassium was subsequently discarded in favour of K from kalium). At a later date Berzelius denoted an oxide by dots, equal in number to the number of oxygen atoms present, placed over the element; this notation survived longest in mineralogy. He also introduced barred symbols, i.e. letters traversed by a horizontal bar, to denote the double atom (or molecule). Although the system of Berzelius has been modified and extended, its principles survive in the modern notation.
In the development of the atomic theory and the deduction of the atomic weights of elements and the formulae of compounds, Dalton’s arbitrary rules failed to find complete acceptance. Berzelius objected to the hypothesis that if two elements form only one compound, then the atoms combine one and one; and although he agreed Extension of the atomic theory.with the adoption of simple rules as a first attempt at representing a compound, he availed himself of other data in order to gain further information as to the structure of compounds. For example, at first he represented ferrous and ferric oxides by the formulae FeO2, FeO3, and by the analogy of zinc and other basic oxides he regarded these substances as constituted similarly to FeO2, and the acidic oxides alumina and chromium oxide as similar to FeO3. He found, however, that chromic acid, which he had represented as CrO6, neutralized a base containing 13 the
- ↑ Dalton’s atomic theory is treated in more detail in the article Atom.
- ↑ Berzelius, however, appreciated the necessity of differentiating the atom and the molecule, and even urged Dalton to amend his doctrine, but without success.
- ↑ The following symbols were also used by Bergman:—
 ,
,
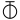 ,
,
 ,
,
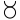 ,
,
 ,
,
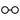 ,
,
 ,
,
 ,
,
 ,
,
 ,
,
which represented zinc, manganese, cobalt, bismuth, nickel, arsenic, platinum, water, alcohol, phlogiston. - ↑ The following are the symbols employed by Dalton:—
 ,
,
 ,
,
 ,
,
 ,
,
 ,
,
 ,
,
 ,
,
 ,
,
 ,
,
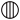 ,
,
 ,
,
 ,
,
 ,
,
which represent in order, hydrogen, nitrogen, carbon, oxygen, phosphorus, sulphur, magnesia, lime, soda, potash, strontia, baryta, mercury; iron, zinc, copper, lead, silver, platinum, and gold were represented by circles enclosing the initial letter of the element.
