as nitrogen. Two methods were adopted for isolating this gas. One was a repetition of a process which had been employed by Cavendish in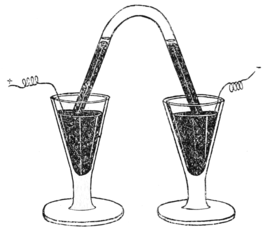 Fig. 1.1785; it consisted in passing electric sparks for a long time through air, confined over mercury, in presence of caustic alkali. The accompanying woodcut gives an idea of the apparatus he employed. This experiment had been made by Priestley ten years previously, but not with quantitative accuracy. It was Cavendish's object to inquire whether the nitrogen of the atmosphere had any claim to be regarded as a homogeneous substance, but he left the question undecided. Having continued to pass sparks through a measured volume of air, with the occasional addition of oxygen, until no
Fig. 1.1785; it consisted in passing electric sparks for a long time through air, confined over mercury, in presence of caustic alkali. The accompanying woodcut gives an idea of the apparatus he employed. This experiment had been made by Priestley ten years previously, but not with quantitative accuracy. It was Cavendish's object to inquire whether the nitrogen of the atmosphere had any claim to be regarded as a homogeneous substance, but he left the question undecided. Having continued to pass sparks through a measured volume of air, with the occasional addition of oxygen, until no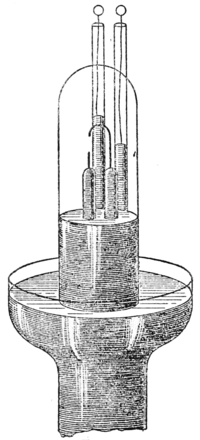 Fig. 2.further diminution in volume occurred, he found that after the excess of oxygen had been removed, the residue amounted to not more than 1/120th part of the whole of the nitrogen. The actual volume of the inactive gases in the nitrogen of the atmosphere is one eighty-fourth. Cavendish did not pursue the investigation further, and the discovery of argon was postponed for a century.
Fig. 2.further diminution in volume occurred, he found that after the excess of oxygen had been removed, the residue amounted to not more than 1/120th part of the whole of the nitrogen. The actual volume of the inactive gases in the nitrogen of the atmosphere is one eighty-fourth. Cavendish did not pursue the investigation further, and the discovery of argon was postponed for a century.
A convenient form of apparatus for repeating Cavendish's experiment is shown in the accompanying figure. The gas, air mixed with oxygen, is confined over mercury in an inverted test-tube, in contact with a few drops of a solution of caustic potash; and by connecting the rings with wires from the secondary coil of an induction apparatus, sparks pass between the platinum terminals in the interior of the test-tube. The volume of the gas rapidly diminishes; and in a few hours, the gas is removed to a clean tube, and the excess of oxygen absorbed by burning phosphorus; the inert gases remain behind. On a larger scale, the apparatus used by Lord Rayleigh, consisting
