Popular Science Monthly/Volume 21/July 1882/The Mechanics of Intermittent Springs
| THE MECHANICS OF INTERMITTENT SPRINGS. |
By Dr. OTTO WALTERHÖFER.
THE springs called thermal springs are found in all latitudes, at various elevations above the sea, and in most of the geological formations. The word thermal does not, however, denote a spring of any particular degree of temperature, and is far from signifying that the springs to which it is applied are all equally warm; for any spring is thermal, the water of which is warmer than the mean annual temperature of the place where it occurs. In the equatorial regions, where the mean annual temperature is about 80°, a thermal spring should have a temperature of about 85°, while in the northern parts of the earth, as, for example, at Yakutsk, in Siberia, where the year's temperature does not exceed 13°, it need be only a little above that. The waters of thermal springs maintain an equable temperature, and must therefore come out of depths in the earth at which the variations in the temperature of the air exert no influence. According to Boussingault, this depth in the tropics is only a little more than one or two feet, but between 48° and 52° of north latitude it is between sixty-six and ninety-three feet below the surface. Besides the springs that are called thermal, many springs are found the temperature of which exceeds the highest mean temperature of the year, and are called warm springs. Examples are the spring at Carlsbad, 167°; that of Wiesbaden, 158°; those of Baden-Baden, 154° to 111°, etc. The depth from which the waters come may be approximately calculated by the rule that the temperature increases one degree for every ninety feet below the surface. Hence the water of the bubbling spring at Carlsbad is supposed to come from a depth of seven thousand three hundred feet.
A third class of springs, the boiling springs, geysers, or hot springs, whose temperature is near the boiling-point of water, are peculiar in respect to the places where they appear. They are found only in volcanic regions; are numerous in Iceland, where there are more than a hundred of them; on the North Island of New Zealand, where they are most abundant in the neighborhood of the Roto Mahana, or Hot Lake; and near the Yellowstone Lake, the Fire-hole and the Madison Rivers, in the region of the Wind River Mountains, in the United States, where some eight hundred of them are grouped within a certain well-defined area.
Among the hot springs, those which are intermittent, or the flow of which is uneven, are regarded with particular interest. Their waters are of a crystal clearness, with a slight tinge of green, and contain in solution considerable quantities of silicic acid, which frequently is deposited, in consequence of the evaporation of the water or the lowering of its temperature, as sinter. The most thorough investigations of the phenomena of intermittent springs have been made at the great geyser at the foot of the Bjärnafell in Iceland. Sartorius von Waltershausen,[1] Descloiseaux,[2] and Bunsen,[3] have made extensive observations upon them, from the results of which, and of their own observations, Mackenzie, Bunsen, and, more recently, O. Lang, have formed theories respecting the mechanical causes of the intermittent flow.
The Great Geyser is situated at a height of one hundred and ten metres (three hundred and fifty-seven feet) above the sea. The part accessible to observation consists of a straight cylinder, R, lined with siliceous sinter, S, Fig. 1, about three metres (or ten feet) in diameter, and twenty-three and a half metres (seventy-six and a half feet) deep, which widens out above into a tunnel-shaped basin, B, from two to three metres (six and a half to ten feet) deep, and seventeen to twenty metres (fifty-five to sixty and one half feet) in horizontal extent. The 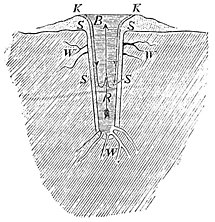 Fig. 1. walls of the basin are formed of a, cone of siliceous sinter, S, from six to nine metres (twenty to thirty feet) high, and about sixty-five metres (more than two hundred feet) broad. The water of the geyser is crystal-clear, with a greenish tint, and flows, except immediately after an eruption, steadily over the rim of the basin. A double motion may be perceived in the column of water inside of the cylinder. A hot stream ascends from the bottom in the axis of the cylinder, while the cooler water descends by the sides, but only to about half-way down, for it there unites with the rising hot current and ascends again to the surface. The temperature of the water is 179° at the surface of the basin; rises at the bottom of the basin to 192, and rises in the bottom of the cylinder to 251° and 260°. The peculiarity of the intermittent streams consists in the fact that their waters mixed with steam are forcibly thrown up in huge jets at longer or shorter intervals. At the Great Geyser, jets of steam, accompanied by detonations raising the water to the height of about ten feet, appear in different parts of the basin at intervals of from one and a half to two hours. At intervals of from twenty-four to thirty hours, a column of water, mixed with steam, rises over the whole extent of the cylinder and the basin, with a sound as of distant thunder, to a height of from twenty-five to thirty metres (eighty to ninety-three feet), and scatters clouds of spray. After an eruption of this kind, the duration of which is about ten minutes, the basin and cylinder are empty; they are gradually filled up again in the course of from four to six hours, after which the processes described above are repeated. The loss of water occasioned by the eruption and overflow is made up for by side-streams, a part of which, W, being near the surface, probably bring in cold water; others, W’, entering at the bottom, water of a higher temperature than the boiling-point.
Fig. 1. walls of the basin are formed of a, cone of siliceous sinter, S, from six to nine metres (twenty to thirty feet) high, and about sixty-five metres (more than two hundred feet) broad. The water of the geyser is crystal-clear, with a greenish tint, and flows, except immediately after an eruption, steadily over the rim of the basin. A double motion may be perceived in the column of water inside of the cylinder. A hot stream ascends from the bottom in the axis of the cylinder, while the cooler water descends by the sides, but only to about half-way down, for it there unites with the rising hot current and ascends again to the surface. The temperature of the water is 179° at the surface of the basin; rises at the bottom of the basin to 192, and rises in the bottom of the cylinder to 251° and 260°. The peculiarity of the intermittent streams consists in the fact that their waters mixed with steam are forcibly thrown up in huge jets at longer or shorter intervals. At the Great Geyser, jets of steam, accompanied by detonations raising the water to the height of about ten feet, appear in different parts of the basin at intervals of from one and a half to two hours. At intervals of from twenty-four to thirty hours, a column of water, mixed with steam, rises over the whole extent of the cylinder and the basin, with a sound as of distant thunder, to a height of from twenty-five to thirty metres (eighty to ninety-three feet), and scatters clouds of spray. After an eruption of this kind, the duration of which is about ten minutes, the basin and cylinder are empty; they are gradually filled up again in the course of from four to six hours, after which the processes described above are repeated. The loss of water occasioned by the eruption and overflow is made up for by side-streams, a part of which, W, being near the surface, probably bring in cold water; others, W’, entering at the bottom, water of a higher temperature than the boiling-point.
The following theories have been advanced in explanation of the periodical eruptions of the Great Geyser, and of the phenomena of intermittent springs generally:
According to Mackenzie's theory, a hollow space, b, Fig. 2, exists in the interior of the earth wherever an intermittent spring occurs, the walls of which are formed of thick stone, free from penetrating clefts. Water-ways entering it from above and from the sides bring in cool waters; while other streams bring up from below water heated to above the boiling-point, or steam from the volcanic foci. The inferior conducting power of the rock-masses, inclosing the chamber

Fig. 2.—Mackenzie's Theory of Eruption.
and the stream-channels, prevents any material diminution of the temperature of the steam and the water, so that the chamber, b, becomes filled with hot water and steam. By virtue of its levity, the latter collects in the upper part of the chamber, while the water covers the bottom, and at the same time, as it is added to by the constant inflow from the canals which enter the chamber, b, rises and shuts up the tube at a. The steam is thus deprived of an outlet, and, since it is continually compressed into a smaller space by the constantly increasing mass of water, is added to by the entrance of new steam, and is heated to a higher temperature by the heat brought in with the hot water that keeps flowing in; it on its side exercises upon the surface of the water in the chamber a pressure that, increasing every moment, gradually raises the water in the tube, c, and causes an overflow over the rim of the basin, d. Finally, the pressure of the inclosed steam becomes so powerful as to overcome the absolute weight of the mass of water in the tube, c, and throw it up strongly and suddenly in fountain-like spouts. After the steam in b has relieved itself, and the pressure on the water has thereby been diminished, a becomes again closed up by the rushing back of the water from the tube and the flow of water from the chamber, and the conditions requisite to another eruption are produced. The temperature of the water in the chamber and in the tube rises continually between one eruption and another, and reaches in many places the degree at which steam is formed. This is the case in the lower part of the tube at a, where, the water nearer the chamber having a higher temperature than in other parts of c, the steam rises in c and causes the bubblings which appear in the basin at intervals of from one and a half to two hours. This theory, which supposes a subterranean cavity acting as a kind of steam-boiler, has now very few adherents, since Bunsen has given an explanation of the phenomenon that makes the supposition of a cavity in the interior of the earth unnecessary.
Bunsen gave his sagacious explanation of the periodical eruptions of the Great Geyser after observations and researches which he himself undertook in the year 1846. He found that the temperature in the geyser-tube, R, Fig. 1, is in a state of continuous increase between one eruption and another. Thus, if the whole column of water, R, is divided into layers of a specified thickness, one of these layers, for example, at a certain depth below the surface would show immediately after an eruption a temperature of 187°, which would rise after an interval to 188°, and then to 189°, etc. The more deeply situated layers would have a higher temperature at first, which would increase in the same manner. In no layer, however, would the temperature at which water is changed into steam be indicated before an eruption. The passage of water into steam—that is, its boiling—does not take place under all circumstances at 212°, but only when the pressure on its surface is equal to the weight of one atmosphere, or fifteen pounds to the square inch. If the surface in question is exposed to a lower or a higher pressure than this, water will boil at a correspondingly lower or higher temperature. Thus the boiling-point is depressed as we ascend mountains and enter regions where the pressure of the superincumbent atmosphere is less than fifteen pounds to the square inch. Conditions also exist in nature in which the boiling-point is raised to more than 212°, and may be found, for example, in the intermittent springs. To return to the tube R in the Great Geyser (Fig. 1), the water in which we have divided into a number of horizontal layers: a greater weight is put upon each successive layer in the descending series, since each one has to bear the weight of all the layers above it in addition to that of the atmosphere. Water, the temperature of which exceeds 212°, is called superheated. It has the property of being convertible instantly into steam as soon as the weight laid upon it is removed. Bunsen turned this physical behavior of water to the explanation of the eruptions of the Great Geyser. The water brought up in the streams (W', Fig. 1) is in a superheated condition on account of the depth from which it comes and the pressure to which it is exposed. It is not, however, converted into steam on reaching R, because that is prevented by the weight of the column of water above. The layers of water above it are, however, heated by convection from it, so that they become specifically lighter, and originate the axial current toward the surface. As soon as it reaches the surface, the water is cooled by radiation, and a part of it falls back in the shape of the downward currents along the sides of the tube, causing a depression of temperature, and a corresponding delay in the formation of steam. But these currents are weak, their water is gradually becoming warmer as it gets farther from the surface, so that they only reach about the middle of the tube R, and fail to prevent the temperature of the whole water-column from finally rising under the influence of the constant flow of superheated water from the streams, W’. The strata of water in the middle of the tube finally reach the temperature of the boiling-point at that depth. This water is then converted into steam, and thereby the pressure upon the lower strata is diminished. Then the strata of water still deeper in the tube are also converted into steam, and this throws the masses of water above it energetically out of the geyser-tube. The water being cooled somewhat in the air, a part of it falls back in the tube, and, producing a reduction of temperature, causes a short interruption of the formation of steam, but that is resumed again as the superheated water flows in, and continues till the whole column has been so reduced in temperature, by the water that falls back, that the liquid strata no longer reach the boiling-point corresponding with the pressure upon them, and the eruption ceases.
According to the theory of Lang, the Great Geyser occupies a space in the interior of the earth in shape somewhat like Fig. 3. The tube

Fig. 3.
R, by which the waters are connected with the surface, is bent upward at x, to be bent downward again at y into the tube Z, which, reaching the depths of the earth, is connected with the channels V. These channels conduct to Z hot water mixed with steam, while R is supplied by the streams S, which lie near the surface, with cold water. The geyser-tube becomes stopped at x by the accumulation from these two sources, and the steam rising from Z, deprived of an outlet, collects at y. Its elasticity being augmented by the masses of steam that keep coming up, and by the continued accession of heat from Z, it forces away the water that is in contact with it, and gradually fills the connecting passage x y and a part of Z, while it interposes a separation between the water-columns R and Z. The water in R becomes heated by contact with the imprisoned steam, and will obviously attain a higher temperature in the lower than in the upper part of the geyser-tube R. Superheated water is produced in R as well as in Z, because the weight of the water in R and the upward pressure of the water, and the confined steam in Z, produce pressure and counter-pressure. As steam and water continue to rise from the channels V, the level of the water in x y is depressed, for the steam can exercise its force only in the direction of R. As the expansive force of the steam increases, a greater quantity of water is driven over the rim of the basin till the force of the steam becomes so great as to exceed the weight of the column of water in R. Single puffs of steam escape, the elastic force of the vapor is slightly diminished, and a sudden development of steam is produced from the superheated water in Z, causing the whole column of water in R to be thrown forcibly into the air. The super-heated water which has got into R during this movement is likewise converted into steam, increasing the effect. The conditions required by the last theory for the origin of an intermittent spring exist in nature. Volcanic forces produce suitable crevices, and the water, leaving siliceous deposits upon the walls, makes these clefts steam-tight.
The time which elapses between two successive eruptions varies in different intermittent springs. It depends upon the length and breadth of the whole geyser-shaft, and the distance to which it penetrates toward the interior of the earth. The temperature of the superheated water and the amount of steam that is formed from it are also largely dependent on the size of the spring. Thus, Strokr and the Little Geyser, intermittent springs in Iceland, also lying at the foot of Bjärnafell, have much stronger eruptions than the Great Geyser. The intermittent springs of the North Island of New Zealand are distinguished by their beautiful snow-white deposits of siliceous sinter, within which the water of the basin appears blue. The most imposing of known geysers are those of North America, of which the Giant shoots its jet to the height of two hundred and fifty feet.