Popular Science Monthly/Volume 7/May 1875/The Chemical Radiations
| THE CHEMICAL RADIATIONS. |
By W. J. YOUMANS, M. D.
WITH that proneness to go wrong, which we notice in most things human, and which crops out in science as well as elsewhere, the art of making pictures by the chemical action of radiant forces has got a false name. This is all the worse, as it was at first correctly designated, and that too by him who had the clearest right to give the process a title. Davy and Wedgwood, early in the century, had labored to produce sun-pictures by means of the camera-obscura but had met with little success. In 1814 M. Neipce, of Chalons, in France, took up the subject, and, in the course of ten years' assiduous work, he succeeded in a method of forming sun-pictures on chemically-prepared copper, pewter, and glass plates, by which the lights, semi-tints, and shadows, were represented as in Nature, and he also succeeded in making the impressions lasting. In 1827 he sent a paper to the Royal Society, accompanied with specimens; but, as he kept the process a secret, the communication could not be received. The process, however, he named heliography, or sun-drawing, a term by which it was truthfully characterized. M. Daguerre, another Frenchman, had been working at the same problem, and in 1829 these two men, with a common purpose, formed a partnership to carry on their researches jointly. Neipce died before the work was matured, and Daguerre, very naturally, reaped the honor of it. The French Government bought his secret, paying with a life-pension, and promulgating it to the world, without restriction of patent, in August, 1839. The new pictures were at once known as daguerreotypes, and the mode of making them the daguerreotype process. These uncouth terms endured for a while, but were at length supplanted by the word photography, or light-drawing, which has become established. Yet the appellation is incorrect, and the error is as broad as the difference between light and darkness. It is not light that makes the picture, but dark radiations that are associated with it, and that have the peculiar effect of producing changes in certain chemical compounds.
Although photography, in its wonderful development as an art, belongs to the past generation, yet the knowledge of the chemical effects ascribed to light is as old as chemical science. The subject began to be inquired into, experimentally, about 100 years ago. In fact, like most other modern chemical results, it had not escaped the notice of the alchemists, but, like every thing else they discovered, it was subordinated to their mystical speculations. In the multiplicity of their manipulatory processes they stumbled upon a combination which they called luna cornua, or horn-silver, and which is now known as silver chloride. The alchemists knew nothing of its composition, but only that there was silver in it which had undergone a change. They noticed, however, that when this horn-silver was exposed to light it underwent a blackening, and, as they taught that "silver only differed from gold in being mercury interpenetrated by the sulphurous principle of the sun's rays," they concluded that this change, effected by light, was the commencement of the process by which silver was to be transmuted into gold.
It was in 1777 that the illustrious Swedish chemist, Scheele, published the first results of investigations upon the subject undertaken simply for the extension of chemical knowledge. He found that, when powdered horn-silver is spread over paper, and the colors of the solar spectrum are made to fall upon it, the powder in the violet ray turns black sooner than that exposed to the other colors. Senebier afterward showed that the silver chloride was darkened in the violet ray in fifteen seconds to a shade which required the action of the red ray for twenty minutes; that is, the chemical intensity of the violet ray was eighty times greater than the red.
The next step was one of great scientific importance, indicating, not only the differentiation of the different modes of action in the sun-beam, but the actual separation and isolation of the different agents. This took place just at the opening of the present century. It was shown by Sir William Herschel, in 1800, that, when the sunbeam is decomposed by a glass prism, as shown in Fig. 1, the heat is distributed

Fig. 1.—Positions of the Three Spectra.
unequally through the series of colors—is lowest in the violet, increases in the yellow, but is most intense in the red. This he determined by the use of delicate thermometers, and, in the same way, he proved that the thermal rays of the sunbeam are not all thrown into the visible spectrum, but are of such low refrangibility that they accumulate in the dark space below the red. There is therefore a spectrum of dark rays, producing heating effects, which, beginning at A Fig. 1, increases in strength till it approaches the red, and then fades away in the upper region of the spectrum.
These results of Herschel were followed by the discovery of Ritter, made the next year (1801), that the chemical rays, which had been shown to be most active in the violet portion of the spectrum, were also thrown by refraction into the dark space beyond the violet. As a thermometer was the test in the case of heat, so an appropriate chemical substance has to be used to test the distribution of this force. If a solution of silver nitrate is washed over a large sheet of paper, which is then placed upon the wail or screen so as to receive the spectrum upon its surface, and is also made to cover the space considerably above it, a transformation occurs where the radiations fall, producing a blackening which defines the outline of the chemical spectrum. It is now found that the chemical rays are more refrangible than the luminous, and that, while the darkening takes place in the colored spectrum, it it strongest in the violet of all the colors, and extends also through the dark space up to B, as shown in the figure.
It is now exactly 200 years since Newton published his "Optics," in which was described the capital experiment of resolving white light into its constituent colors by the prism. It was the first great step toward showing that what was regarded us perfectly simple turns out to be inexhaustibly complex; and every succeeding step of research, while clearing up some points, has led to others which are still unresolved. One thing, however, seems to be quite clear: the mode of action throughout the spectrum is fundamentally the same. There are three spectra, one of which, the thermal, takes action upon all kinds of matter; another of which, the luminous, acts only upon a certain special form of nerve-matter; while a third, the chemical, produces changes in certain compounds. Although the luminous force acts only upon the nerve of the eye to stir up a sensation, yet we know how infinitely complex and varied is the world of color that results. There is evidence that the dark thermal and chemical radiations are of equal variability and complexity, yet there can be no doubt that all these multitudinous effects are due to a single mode of action. The difference between the thermal and the chemical rays is simply the difference between the red and the green; that is, a difference of wavelength and degree of vibration.
The unequal distribution of the forces of the spectrum is well illustrated by Fig. 2. The middle curve shows the varying intensity of the luminous force. The maximum is at B, in the yellow space; and from this point the intensity of the light rapidly declines each way, its extent being shown by the space shaded with oblique lines. The curve A, with the vertical lines, represents the position and varying force of the heat; and the curve C, horizontally shaded, exhibits the distribution and unequal energy of the chemical force. The three maxima are widely separated as if there were some antagonism among them, and it is noticeable that where the light is strongest the chemical force quite disappears. Different prisms give somewhat different effects but do not change their order.
It thus appears that, so far from light being the agent which produces sun-pictures, the intensest light is powerless upon the chemically prepared plate. It looks as if the illumination neutralized or extinguished the chemical energy. Nevertheless, light and the chemical force are so intimately associated in reflection and refraction that the colors become the guides of the artist in conducting his processes. When a person sits before the operator's camera, ready to be "taken," the radiations which are reflected from his face into the instrument, and collected to a focus by the lens, form three pictures, one behind the other, the thermal, the luminous, and the chemical image. The luminous image is visible upon the ground-glass plate, giving all the

Fig. 2.—Intensities of the Forces of the Spectrum.
colors of the object, but the chemical image is now blurred, and the focus has to be readjusted so that the chemical picture will be clearly and sharply defined; but, as this image is invisible to the operator, be has to make his readjustments by rule. As he cannot reproduce the colors in the photograph, he has to substitute for them tints and shades; but the chemical force is so unequal in the different colors that the natural effects of gradation in tone and shade are not brought out in the picture. This is one of the embarrassments of the process. From the representation In Fig. 2, we should infer that blue colors would act energetically upon the photographic plate and the yellow and red feebly, or not at all, because the chemical rays abound in the former and are absent in the latter. Of this false working of lights Prof. Vogel says: "Blue generally works clear, yellow and red work like black. The yellow freckles appear, therefore, in a picture as black spots, and a blue coat becomes perfectly white. Dark-blue flowers on a light-yellow ground produce in photography light flowers on a dark ground. Red and also fair golden hair become black. Even a very slight yellow shade has an unfavorable effect. A photograph from a drawing is often blemished by little iron-mould specks in the paper, invisible to the eye. These specks frequently appear as black points. There are faces with little yellow specks that do not strike the eye, but which come out very dark in photography. A few years ago a lady was photographed in Berlin whose face had never ])resented specks in photography. To the surprise of the photographer, on taking her portrait, specks appeared that were invisible in the original. A day later the lady sickened of the small-pox, and the specks, at first invisible to the eye, became then quite apparent. Photography in this case had detected, before the human eye, the pock-marks, very feebly tinged yellow." The chemistry of light first became, in the full sense, a branch of science capable of thorough investigation when Dr. Draper devised a method of measuring the force of the chemical rays, and thus brought the subject within the sphere of quantitative research. He showed that these rays are absorbed in the chemical combination, and that the rate of absorption corresponds to the amount of chemical change. He applied mixtures of chlorine and hydrogen gases for this purpose, which combine under the action of the chemical radiations, the measurable rate of combination becoming the index of radiant activity. Professors Roscoe and Bunsen subsequently employed sensitive papers which were blackened in certain times to certain shades, as measurers of the chemical force, and these were used at the Kew Observatory,
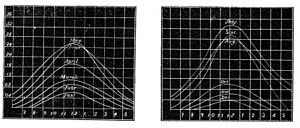
Fig. 3 and 4.—Variation of Chemical Rays at Kew.
near London, to trace the variations of chemical activity in the solar rays. For, as the chemical force is not light, neither does it follow the laws of light in producing its effects. Dr. Draper had previously shown that, as we go southward toward the equator, and the light increases in brilliancy, there is an increasing interference with the chemical rays, the yellow space of no-chemical action widening with the progress southward. It is also well known that there is much greater difficulty in obtaining good photographic pictures under the full blaze of a tropical sun than in our own latitude. The investigations at Kew were accordingly directed to the variations that the chemical rays undergo at different hours of the day and at different seasons of the year. The graphic diagrams, Figs. 3 and 4, show the results that were arrived at in 1866. The curves exhibit the rise and fall of the average monthly chemical intensity with the hour of the day, from 6 a. m. to 6 p. m., throughout the year. We see from these curves that the maximum of chemical action occurs at twelve o'clock, and that the forenoon rise and afternoon decline are very nearly equal, while the chemical intensity of July is fully seven times as great as in December.
The statements that have been made that in Mexico, where the light is very intense, from twenty minutes to half an hour is required to produce photographic effects which in New York require only a minute; and the further statement of travelers, engaged in copying the antiquities of Yucatan, that they frequently have been obliged to abandon the use of the camera, and take to their sketch-books, have led to some investigations, similar to those at Kew, for determining the intensity of the chemically-active rays in the tropics. Prof. Thorpe experimented at Pará, situated nearly under the equator, in the northern province of the Brazils, and lying on a branch of the Amazon. Of the results. Prof, Roscoe remarks: "Owing to the rainy sear son having commenced when the experiments were made, the changes in the chemical intensity, as observed from hour to hour, and even from minute to minute, are very sudden and remarkable; this is well shown by the zigzag lines of Figs. 5 and 6; and these, compared with the dotted lines below, indicating the corresponding action on the same

Figs. 5 and 6.—Variation of Chemical Rays in the Tropics.
day at Kew, show the enormous variation in chemical intensity which occurs under a tropical sun in the rainy season. Regularly every afternoon, and frequently at other hours of the day, enormous thunder-clouds obscure the sky, and, discharging their contents in the form of deluging rain, reduce the chemical action nearly to zero. The storm quickly passes over, and the chemical intensity rapidly rises to Its normal value. By comparing the curves for Pará and Kew on the same days, we obtain some idea of the energy of chemical action at the tropics, and it is at once evident that the alleged failure of the photographer cannot at any rate be ascribed to a diminution in the sun's chemical intensity, which, in the month of April, 1866, was nearly seven times as great at Pará as at Kew."