Diseases of Swine (8th edition)/Chapter 23
Swine pox (SwP) was first reported in 1842 by Spinola in Europe, and in 1929 by McNutt et al. in North America. Worldwide in distribution, SwP is usually associated with swine operations that have poor sanitation and/or intensive breeding with open-herd management. SwP causes little economic loss and can usually be differentiated from other diseases by its clinical signs and epidemiology.
The clinical signs of infection may be caused by either vaccinia virus (VV) or SwP virus (SwPV) (Manninger et al. 1940; Shope 1940). Since the eradication of smallpox (variola) and the subsequent cessation of vaccinia vaccination and VV infection of swine, the occurrence of SwP has decreased (Meyer and Conroy 1972).
ETIOLOGY[edit]
SwPV is classified in the family Poxviridae and is the only member of the genus Suipoxvirus (Francki et al. 1991). The large virion (300-450 × 176-260 nm) contains double- stranded DNA in a characteristic poxvirus core or nucleoid bordered by lateral bodies and surrounded by an outer coat of numerous proteins. Because SwPV is enveloped, it is ether sensitive (Cheville 1966a). Typical of poxviruses, it shares antigenic epitopes with members of other poxvirus genera (Ouchi et al. 1992). The large genome has room for many insertions, and the SwPV has been considered a vector for the delivery of immunogens to swine (Foley et al. 1991).
The virus, once adapted to grow in cell culture, readily reaches high titers and maintains its pathogenicity for swine (Kasza et al. 1960; Kasza and Griesemer 1962).
EPIDEMIOLOGY[edit]
SwP is a disease of pigs only; SwPV does not infect cattle, horses, sheep, dogs, cats, domestic fowl, rabbits, guinea pigs, rats, mice, or humans (Shope 1940; Schwarte and Biester 1941; Datt 1964).
The pig louse (Haematopinus suis) serves as a mechanical vector and is considered the primary means of transmission of SwPV (Shope 1940). The ventral distribution of pox lesions can be correlated to the habitat of H. suis. Flies and mosquitoes have been implicated as mechanical vectors, and in this situation, pox lesions are distributed on the backs and sides of affected pigs (Schwarte and Biester 1941).
Occasionally, horizontal transmission may occur in the absence of insects, since SwPV is shed in nasal and oral secretions and from lesions. The SwPV is present in infected epithelium and in dry scabs produced in the later stages of infection. Abraded skin can serve as the route of entry for SwPV. The virus may persist for up to a year in the desiccated form, likely accounting for the persistence of SwP in affected herds.
The morbidity of SwP may approach 100% in young stock up to 4 months of age where poor hygiene occurs. The disease may have a seasonal incidence related to the prevalence of insects. Mortality is usually less than 5%.
CLINICAL SIGNS[edit]

SwP is a typical pox disease, with the development of the lesions progressing through the classic stages of macule (reddening), papule (reddening with edema), vesicle (fluid exuding from the pox lesion), and pustule or crust formation (drying of the lesion with scab formation) (Fig. 23.1). The vesicular stage is not readily observed in SwP. The time from macule formation to scabbing and resolution of lesions is 3-4 weeks. A lymphadenitis may occur. Complication with bacterial infection prolongs the resolution of the lesion (Miller and Olson 1978, 1980).
Young animals are more severely affected than adults. Suckling piglets may have lesions in the perioral epithelium or a generalized disease with lesions all over the body. Lesions are more prominent on the hairless parts of the skin. Mechanical transmission by pig lice results in lesions on the lower parts of the body, including the udder and vulva. Transmission by flies and mosquitoes is less common and may result in lesions on the dorsal parts of the body, including the snout and ears. Sporadic congenital infection has been reported (Borst et al. 1990; Paton et al. 1990). The incubation period following experimental intradermal inoculation is 2-5 days.
The incubation period following intravenous inoculation is 10-14 days (Shope 1940) but can be as short as 5 days (Kasza and Griesemer 1962), the range likely being related to the inoculation dosage. Incubation under field conditions may be up to 14 days.
PATHOGENESIS[edit]
SwP is usually initiated by SwPV entering a break in the skin such as a louse bite or an abrasion. The SwPV replicates in the cytoplasm of the cells of the stratum spinosum. Lesions in local lymph nodes are not extensive, and the virus is not readily isolated from them. Secondary lesions are believed to result exclusively from the spread of the virus from an existing lesion, not by a cellassociated viremia as in most other poxvirus infections. The virus has not been recovered from the blood (Kasza and Griesemer 1962; Shope 1940), but this may be due to the fact that the SwPV is difficult to isolate, especially when in low concentrations. Viremia must occur, as evidenced by congenital infection in piglets.
Following intravenous inoculation, gnotobiotic piglets developed skin lesions uniformly over their entire bodies but not in internal organs (Meyer and Conroy 1972), confirming the propensity of SwPV to infect the integument. In gnotobiotic pigs, the macular stage was not observed; therefore, the early reddening may be the combined effect of reaction to louse bites, opportunistic bacteria, and SwPV.
LESIONS[edit]
The gross lesions of SwP are described in the section on clinical signs.
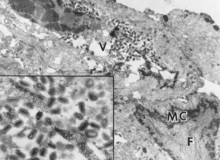
The histopathological changes caused by SwPV are similar to those of other poxviruses (Kasza and Griesemer 1962; Cheville 1966a, b; Conroy and Meyer 1971; Meyer and Conroy 1972; De Boer 1975). The virus replicates in the stratum spinosum, causing hydropic degeneration. Sections stained with hematoxylin and eosin reveal one to three eosinophilic-staining intracytoplasmic inclusion bodies (ICIBs) in some cells during all stages of the infection. Characteristic central nuclear clearing, which is also observed with capripoxvirus infection of sheep, goats, and cattle, occurs in infected epithelial cells containing ICIBs and is a result of margination of chromatin with nuclear filamentous matrix formation. There may be intercellular edema, which could account for the transient vesicular stage. Acantholysis, the separation of individual cells of the stratum spinosum with loss of desmosomes and detachment into the vesicular fluid, was not found with SwPV infection (Meyer and Conroy 1972). Necrosis of epithelial cells and the presence of neutrophils and macrophages in the dermis and epidermis are common in later stages of the pox lesion (Fig. 23.2). The pustules involve the full thickness of epithelium and may heal, leaving a light scar. Poxvirus particles can be observed in the cytoplasm of infected cells by electron microscopy (Fig. 23.3).
DIAGNOSIS[edit]
SwP is typified by the presence of pox lesions on the skin of the belly, ears, snout, vulva, and back. Secondary bacterial infections may cause more extensive lesions and local abscess formation. Diseases to consider in the differential diagnosis of SwP include classic vesicular diseases, bites from needle teeth, pityriasis rosea, parakeratosis, parasitic skin disorders, allergic skin reactions, early stages of ringworm, staphylococcal or streptococcal epidermitis, and cutaneous erysipelas.
The SwPV infection may be confirmed by detection of viral antigens using immunofluorescence and electron microscopy. The presence of ICIBs along with central nuclear clearing in affected epithelial cells is pathognomonic for SwPV, as VV infection does not cause the central nuclear clearing (Teppema and De Boer 1975). The virus may be isolated by multiple passages (up to five) in swine cell cultures and definitively identified by neutralization with specific-reference antiserum.

Serologic diagnosis utilizes the agar gel immunodiffusion test or the more sensitive counterelectrophoresis test (De Boer 1975). Swine do not develop high levels of neutralizing antibody (Shope 1940; Kasza et al. 1960), and negative results on the virus neutralization test should not be interpreted as the absence of SwPV infection.
TREATMENT[edit]
There is no known treatment for SwP, although antibiotics may diminish the complications caused by infection with secondary bacteria. Affected animals should be isolated. Ectoparasites should be controlled by the use of licensed insecticides, and the premises should be aggressively cleaned and disinfected.
PREVENTION[edit]
Vaccines for SwP have not been developed due to the relatively low economic importance of this disease. Recovered animals are specifically immune to SwP (Shope 1940) even in the absence of demonstrable neutralizing antibody, implying the importance of cell-mediated immunity.
Animals introduced into herds should be carefully inspected for skin lesions and ectoparasites. Good hygienic practices are essential for the prevention and control of SwP.
REFERENCES[edit]
Borst, G. H. A.; Kimman, T. G.; Gielicens, A. L. J.; and Van der Kamp, J. S. 1990. Four sporadic cases of congenital swinepox. Vet Rec 127:61-63.
Cheville, N. F. 1966a. The cytopathology of swinepox in the skin of swine. Am J Pathol 49:339-352.
___. 1966b. Immunofluorescent and morphologic studies on swinepox. Pathol Vet 3:556-564.
Conroy, J. D. And Meyer, R. C. 1971. Electron microscopy of swinepox virus in germfree pigs and in cell culture. Am J Vet Res 32:2021-2032.
Datt, N. S. 1964. Comparative studies of pigpox and vaccinia viruses. I. Host range pathogenicity. J Comp Pathol 74:62-80.
De Boer, G. F. 1975. Swinepox: Virus isolation, experimental infections and the differentiation from vaccinia virus infections. Arch Virol 49:141-150.
Foley, P. L.; Paul, P. S.; Levings, R. L.; Hanson, S. K.; and Middle, L. A. 1991. Swinepox virus as a vector for the delivery of immunogens. Ann NY Acad Sci 646:220-222.
Francki, R. B.; Faquot, C. M.; Knudsen, D. L.; and Brown, F. 1991. Classification and Nomenclature of Viruses. Fifth Report of the International Committee on Taxonomy of Virus. Arch Virol, Suppl 2. New York: Springer Verlag, pp. 320-326. Kasza, L., and Griesemer, R. A. 1962. Experimental swine pox. Am J Vet Res 23:443-450.
Kasza, L.; Bohl, E. H.; and Jones, D. O. 1960. Isolation and cultivation of swinepox virus in primary cell cultures of swine origin. Am J Vet Res 21:269-273.
Manninger, R.; Csontos, J.; and Salyi, J. 1940. Über die Atiologie des pockenartigen Ausschlags der Ferkel. Arch Tierheilkd 75:159. McNutt, S. H.; Murray, C.; and Purwin, P. 1929. Swinepox. J Am Vet Med Assoc 74:752. Meyer, R. C., and Conroy, J. D. 1972. Experimental swine pox in gnotobiotic piglets. Res Vet Sci 13:334-338.
Miller, R. B., and Olson, L. D. 1978. Epizootic of concurrent cutaneous streptococcal abscesses and swinepox in a herd of swine. Am J Vet Med Assoc 172:676-680.
___. 1980. Experimental induction of cutaneous streptococcal abscesses as a sequela to swinepox. Am J Vet Res 41:341-347.
Ouchi, M.; Fujiwara, M.; Hatano, Y.; Yamada, M.; and Shiro, N. H. 1992. Analysis of swinepox virus antigens using monoclonal antibodies. J Vet Med Sci 54:731-737.
Paton, D. K.; Brown, I. H.; Fitton, J.; and Wrathall, A. E. 1990. Congenital pig pox: A case report. Vet Rec 127:204. Schwarte, L. H., and Biester, H. E. 1941. Pox in swine. Am J Vet Res 2:136-140.
Shope, R. E. 1940. Swine pox. Arch Gesamte Virusforsch 1:457-467.
Spinola, M. 1842. Krankheiten der Schweine. Ed. A. Hieschwald. Berlin, p. 204. Teppema, J. S., and De Boer, G. F. 1975. Ultrastructural aspects of experimental swinepox with special reference to inclusion bodies. Arch Virol 49:151-163.