Diseases of Swine (8th edition)/Chapter 25
Vesicular diseases in swine are identical clinically. They can be caused by infection with the viruses of foot-andmouth disease (FMD), vesicular stomatitis (VS), vesicular exanthema of swine (VES), and swine vesicular disease (SVD). FMD, VES, and SVD are exotic to the United States, while VS is enzootic. The importance of vesicular diseases in today's world-trade environment remains high. Because FMD is extremely contagious and has such a dramatic economic impact, vesicular diseases must be properly diagnosed, reported, and controlled.
In Italy in 1546, Fracastorius made what is probably the first report of a vesicular disease (Bulloch 1927). FMD was the first animal disease shown to be caused by a filterable agent (Loeffler and Frosch 1898). VS was recognized in horses and cattle in the United States during World War I (Cotton 1927), but infection in swine was not reported until 1943 (Schoening 1943). The first report of VES was in 1932 in California (Traum 1934), although it was initially thought to be FMD. A new vesicular disease, SVD, emerged in Italy in 1966 (Nardelli et al. 1968). San Miguel sea lion viruses (SMSVs), isolated from marine mammals, were shown to cause vesicular disease in inoculated swine (Smith et al. 1973; Berry et al. 1990). The history and distribution of these viruses are given in Table 25.1.
| Virus | Disease First Reported | Agent Identified | Distribution | Areas Free |
|---|---|---|---|---|
| Foot-and-mouth disease | 1546 | 1898 | Sporadic: Europe Enzootic: South America, Asia, Africa |
North and Central America, Australia, New Zealand, Japan |
| Swine vesicular disease | 1966 | 1968 | Europe, Japan, Hong Kong, Taiwan | North, Central, and South America; Africa; United Kingdom |
| Vesicular exanthema of swine | 1932 | 1934 | Last North American case in 1956 | World |
| San Miguel sea lion | None 1973 | Pacific coast of North America | Remainder of world | |
| Vesicular stomatitis | 1916 | 1927 | North, Central, and South America | Remainder of world |
ETIOLOGY
[edit]The characteristics of the viruses that cause vesicular diseases are shown in Table 25.2.
| Disease Agents | Classification | Nucleic Acid | Size and Stability | Proteins/Antigens | Serotypes |
|---|---|---|---|---|---|
| Foot-and-mouth disease virus | Picornaviridae Aphthovirus | SS RNAa (positive sense) | 22–30 nm; labile below pH 7.0; ether-resistant | 4 structural proteins, plus a group of reactive nonstructural proteins | 7: numerous subtypes show varied degrees of cross-protection within each serotype |
| Swine vesicular disease virus | Picornaviridae Enterovirus | SS RNA (positive sense) | 22–30 nm; acid-stable; ether-resistant | 4 structural proteins | 1: related to Coxsackie B-5 virus |
| Vesicular exanthema of swine/San Miguel sea lion viruses | Caliciviridae Calicivirus | SS RNA (positive sense) | 35–39 nm; labile below pH 3; ether-resistant | 1 polypeptide in coat; plus group reactive antigens | 26 or more (13 known for VESVs; at least 13 for SMSVs) |
| Vesicular stomatitis virus | Rhabdoviridae Vesiculovirus | SS RNA (negative sense) | 70 × 170 nm; bullet-shaped; ether-labile; stable at pH 5–10 | 1 immunogenic glycoprotein | 2 of importance to swine: New Jersey and Indiana 1 |
aSS RNA = single-stranded RNA.
Foot-and-Mouth Disease
[edit]FMD viruses (FMDVs) are in the family Picornaviridae and the genus Aphthovirus (Francki et al. 1991c). Aphtha is the Greek word for "vesicles in the mouth." These viruses are small (22-30 nm), nonenveloped, and labile below pH 7.0, and they have single-stranded positivesense RNA with approximately 8450 nucleotides that serve as messenger RNA. The high rate of nucleotide substitutions in the single-stranded RNA viruses accounts for their wide variation (Domingo et al. 1993; Vosloo et al. 1996). Perhaps one of the most important sites for substitutions is the GH loop (codons 133-158) of viral protein 1 (VP1) (Acharya et al. 1989), which is adjacent to the common aphthovirus receptor site RGD (Fox et al. 1989). There are four structural viral proteins, of which VP1 has been shown to elicit protective immunity in cattle (Bachrach 1977). Viral RNA polymerase, a nonstructural protein, has been termed "virus infection associated antigen" (VIAA) for diagnostic purposes but is more accurately termed "FMDV nonstructural protein 3D." One copy of 3D occurs in each viral particle (Newman et al. 1994). The FMDV nonstructural protein 2C is present in clarified FMD vaccines at levels insufficient to elicit an immune response (Lubroth et al. 1996).
There are seven serotypes of FMDV: A, O, C, Asia 1, and South African Territories (SAT) 1, 2, and 3. There is no cross-protection between the serotypes (Brooksby 1982). All serotypes have numerous subtypes, which may arise during acute or persistent infection (Gebauer et al. 1988; Vosloo et al. 1996). The serologic relatedness of subtypes is determined by "r-values," the ratio of antibody titers between new and reference viruses (Pereira 1977).
Swine Vesicular Disease
[edit]SVD virus (SVDV) is in the family Picornaviridae, genus Enterovirus (Francki et al. 1991c). The virions are small (22-30 nm), nonenveloped, and acid-stable. Each has a single-stranded positive-sense RNA and four structural viral proteins. There is one serotype of SVDV, whose closest relative is the human enterovirus Coxsackie B-5 (Graves 1973).
Vesicular Exanthema of Swine and San Miguel Sea Lion Viruses
[edit]The VESVs and SMSVs, members of the Caliciviridae family, are closely related caliciviruses (Smith et al. 1973), named from the Latin calices (cups) for the cupshaped depressions on the virion. The viruses are 35-39 nm in diameter, nonenveloped, moderately acid-stable, and contain single-stranded positive-sense RNA (Francki et al. 1991b). The capsomers are composed of one major polypeptide. There are 13 known serotypes of VESVs, which are sequentially designated (except for strains 1934B and 101) by a letter followed by the year of isolation (e.g., VESV A48).
There are at least 13 SMSV serotypes, which are numbered consecutively (with no SMSV 3, which does not exist) in order of discovery.
Vesicular Stomatitis
[edit]The bullet-shaped VSVs belong to the family Rhabdoviridae, genus Vesiculovirus. The viral particles are 70 × 170 nm, enveloped, ether-labile, and stable at pH 5-10, and they contain a single-stranded, negative-sense RNA. There are five structural viral proteins: L, G, N, NS, and M. The glycosylated G protein confers the type specificity and elicits neutralizing and hemagglutinating antibodies (Francki et al. 1991a). The viral nucleoprotein N and the matrix protein M account for serologic-group cross-reactions. There are at least 11 vesiculovirus serotypes (Tesh et al. 1983; Travassos da Rosa et al. 1984), of which serotypes New Jersey (NJ), Indiana 1, Indiana 2, and Indiana 3 are of importance to livestock.
EPIDEMIOLOGY
[edit]The epidemiological features of the vesicular diseases are summarized in Table 25.3.
| Disease Agent | Main Species Affected | Transmission | Morbidity | Carrier State | Contamination of Meat and By-Products |
|---|---|---|---|---|---|
| Foot-and-mouth disease virus | Swine, cattle, sheep, goats, African buffaloes; some strains are host restricted | Aerosol, contact, fomites | High | Cattle, sheep, goats, African buffaloes | Frozen meat, lymph nodes, bone marrow, milk products |
| Swine vesicular disease virus | Swine, humans | Contact, fomites | Moderate | No | Virus persists in cured meat and gland products |
| Vesicular exanthema of swine/San Miguel sea lion viruses | Swine, various marine animals | Contact, fomites | Moderate | No | Meat |
| Vesicular stomatitis virus | Horses, cattle, swine, humans | Biting insects; some by contact | Moderate to low | No | Not documented |
Foot-and-Mouth Disease
[edit]FMD is considered the most contagious disease of livestock. Essentially all cloven-footed species are susceptible to FMDV. In some texts, the possibility of human infection is stated, but this has yet to be documented. Infected animals shed large quantities of virus into their surroundings for at least 7 days. Aerosol exposure is the major means of spread, particularly in temperate climates, where high humidity and moderate temperatures can favor long-distance airborne spread (Sellers and Parker 1969; Thomson 1994). Tremendous aerosols are generated by swine, which are often called an "amplifier host" (Sellers and Parker 1969; Donaldson and Ferris 1980; Donaldson et al. 1987). Swine may produce up to 108 infectious doses contained in aerosols per day during peak viremia (Sellers et al. 1971), which is up to 1500 times greater than that produced by cattle (Donaldson and Ferris 1980). In hot, arid regions, the disease is mainly spread by direct contact, as aerosols cannot travel long distances due to low humidity and high temperatures. Other means of spread include contaminated semen, meat and milk products, and fomites. Semen from infected cattle contained and transmitted FMDV by artificial insemination (AI) (Cottral et al. 1968), whereas semen from infected swine contained FMDV but did not transmit the disease by AI (McVicar et al. 1978).
The most dramatic proof of carrier buffaloes infecting contact animals was shown by Vosloo et al. (1996). Sporadically over two years, both contact cattle and buffaloes became infected by a cloned SAT-2 virus. The nucleotide sequence varied significantly from the original clone (1.649% nucleotide substitution/year). An inadvertent introduction of a SAT-1 strain infected some buffaloes, and ultimately these carrier animals infected another buffalo. The nucleotide substitution rate for the SAT-1 strain was 1.549% per year. African buffaloes have clearly transmitted FMDV to cattle (Dawe et al. 1994; Hedger and Condy 1985), as well as under experimental conditions (Dawe et al. 1994). The major transmission of FMDV from African buffaloes to cattle occurs when yearling buffalo calves, whose maternal antibody has waned, become infected with virus from carrier buffaloes. It is hypothesized that buffalo calves then shed large quantities of virus and pass the virus to susceptible cattle during close contact or by sharing water holes (Thomson 1995).
Some strains of FMDV may be host restricted under field conditions, as exemplified by the porcinophilic nature of the O1 strain that infected virtually the entire swine population of Taiwan in the spring of 1997. No infections were reported in the 300,000 Taiwanese cattle. Experimentally, O1 Taiwan did not readily infect cattle even when administered in massive doses (Dunn and Donaldson 1997).
On the North American continent, FMD last occurred in Mexico in 1946-54 and in Canada in 1952. It has occurred nine times in the United States, with the last outbreak in 1929. Serotypes A, O, and C occur in South America, and six serotypes (A, O, and C, SAT-1, SAT-2, and SAT-3) occur in Africa. The SAT serotypes are generally restricted to sub-Saharan Africa. Serotypes A, O, C, and Asia 1 occur in Asia and the Middle East. Japan, Australia, New Zealand, and certain Pacific islands are free of FMD. A consistent vaccination program in Western Europe and most of Eastern Europe greatly diminished the prevalence of FMD. The European Union stopped vaccinating for FMD in January 1992. Sporadic local outbreaks occurred in Italy in 1993 (Maragon et al. 1994), in the Balkans in 1995, and in sheep in Greece in 1996. These outbreaks were controlled either by stamping out or by stamping out and vaccination.
Stringent importation regulations (Section 306A of the Tariff Act of 1930 and acts of 1890 and 1903) on animals and animal products have effectively protected the FMD-free status of the United States. The U.S. Department of Agriculture (USDA) conducts research and diagnostic studies with FMDV at its high-containment facilities (greater than biosafety level 3) at the Plum Island Animal Disease Center (PIADC).
FMD results in direct economic losses through the loss of meat, milk, draft power, and genetic stocks, and indirect losses through embargoes and loss of trade. The direct loss from FMD during the first year of an outbreak in the United States was estimated at $4 billion, with indirect costs up to 10 times more (Blackwell 1984).
Swine Vesicular Disease
[edit]SVD is a moderately contagious disease of swine. Mice may be infected experimentally. Persons working with or around infected pigs can harbor SVDV in their nasal passages (Sellers and Herniman 1974), and human infection has been observed (Brown et al. 1976). Swine vesicular disease virus is spread primarily by contact with infected swine or by garbage feeding of SVDV-contaminated meat products. Fecal transmission is not common (Chu et al. 1979; Mann and Hutchings 1980), which is unusual for an enterovirus. The SVDV can survive up to 38 days in a pH range of 3.9-9.1 at refrigerator temperatures (Herniman et al. 1973). Earthworms from soil above pits used to bury infected pigs yielded SVDV when their gut tracts and surfaces were sampled, emphasizing the ability of SVDV to persist in the environment (Coombs 1973). SVDV persisted for 560 days in the lymph nodes of hams prepared from infected swine (Mebus et al. 1993b). The feeding of infected meat represents a disease threat even though the oral route of infection may require at least 300,000 infectious units (Mann et al. 1975; Mann and Hutchings 1980). Abraded mucosa is more readily infected, requiring only about 100 infectious particles.
There is no known carrier state for SVDV. Outbreaks of SVD in Hong Kong (1970) and in England (1972) once defined its eastern and western limits. Recently, SVD has been reported in Italy (1995 and 1996) and in Portugal (1995).
Vesicular Exanthema of Swine and San Miguel Sea Lion Viruses
[edit]VES is a moderately contagious disease. Under certain management situations_namely, the feeding of infected seal meat or contaminated garbage_swine were the only livestock to show clinical VES. Marine animals (fish, sea lions, fur seals, elephant seals, etc.) along the Pacific coast from California to Alaska and likely along the coast of northeastern Russia are apparently the natural hosts of the numerous serotypes of VESVs and SMSVs. A calicivirus was isolated from a California sea lion (Zalophus californianus) from San Miguel Island and designated San Miguel sea lion virus (Smith et al. 1973). Numerous serotypes of SMSVs were isolated from fish and marine mammals (Smith et al. 1977, 1979, 1980, 1983b) and from frozen meat of fur seals fed to mink (Sawyer et al. 1978). Bovine calicivirus (BCV, Tillamook virus), a calicivirus serotype isolated from three dairy calves in Oregon (Smith et al. 1983a), may be of marine origin, as antibodies to BCV were found in various marine mammals (Barlough et al. 1987). Many SMSVs have been shown to produce vesicular disease in experimentally inoculated swine (Smith et al. 1973; Berry et al. 1990). Low levels of antibodies to VESVs and SMSVs have been found in terrestrial mammals (feral swine, foxes, buffaloes, donkeys, and cattle) on the west coast of the United States and on Santa Barbara Channel Islands (Smith and Latham 1978). The relationship of antibodies to natural disease is not clear. The infection of humans with SMSVs is inferred but not corroborated.
The VESVs were shown to be present in the meat of infected swine (Mott et al. 1953) and are spread mainly by feeding of infected meats, contact, or fomites (Bankowski et al. 1955). Long-term carriers have not been shown for VESV.
VES was confined to California from its first description in 1932 until 1951, when pork trimmings from an interstate California passenger train were fed to swine in Wyoming. The ensuing epizootic involved 42 states plus the District of Columbia. The last foci of VES were three large garbage-feeding operations in Secaucus, New Jersey. The epizootic was controlled in 1956, costing $33 million in direct costs and indemnities (Mulhern 1953). Two cases of VES occurred outside the United States. Butcher hogs aboard ships en route to Hawaii in 1946 and 1947 were intercepted and were slaughtered. The second occurrence was in swine fed uncooked garbage from a U.S. military base in Iceland in 1955, which was also promptly controlled (Bankowski 1965). After enactment of laws prohibiting feeding of raw garbage to swine and requiring the slaughter of infected animals, VES was eradicated in the United States. VES was declared an exotic disease in 1959 in the United States, and all countries worldwide remain free of VES.
Vesicular Stomatitis
[edit]VS is considered to be a disease of moderate to low contagious potential, as it is primarily an insect-borne disease. The VSVs can cause clinical disease or subclinical infections in a wide range of animals, including swine, cattle, horses, and humans.
The origin of outbreaks and means of dissemination of VSV is not thoroughly understood. The ecological distribution of VS is river basins and wooded areas, pointing to insect transmission. Serotypes Indiana 1 and NJ have been isolated from sand flies (Lutzomyia shannoni), and transovarial transmission was demonstrated (Tesh et al. 1971; Comer et al. 1990). Sand flies infected with VSV NJ transmitted the virus to suckling mice (Comer et al. 1990). Studies on Ossabaw Island, Georgia, have shown that feral swine become seropositive to VSV NJ during late spring months when insect activity resumes (Stallknecht et al. 1985). Black flies were shown to biologically transmit VSV NJ in their saliva for up to 10 days after feeding on viral fluids (Cupp et al. 1992). Fomites may play a role in the spread of VS in swine. Contact transmission may occur (Schoening 1943) and apparently requires abrasions in the epithelium (Patterson et al. 1955). A carrier state for VSV was not detected by virus isolation or nucleic acid hybridization studies (Redelman et al. 1989).
VS has been recognized in the United States since at least 1916 (Cotton 1927). Vesicular disease outbreaks in cattle and horses in 1925 and 1926 were caused by infection with two distinct serotypes of VSV (Cotton 1927), later called Indiana 1 and New Jersey (NJ). VS was first described in U.S. swine that were used for the production of hog cholera hyperimmune serum in Missouri in 1943 (Schoening 1943). In the United States, epizootics, mostly serotype NJ, occur about every 10-13 years, starting in early summer and ending with the onset of freezing weather. These epizootics generally start in the southwestern states and then spread northward into the Rocky Mountain states. The disease primarily affects horses and cattle, with an occasional spillover to swine. The 1982-83 VS NJ epizootic persisted into winter, causing speculation that VS may be altering its epidemiological pattern. Movement of infected animals extended the scope of this outbreak to Idaho and California. The 1995 outbreak of VS NJ was typical, being confined to western states and disappearing with the onset of cold weather. VS NJ is endemic in the coastal regions of Georgia (Stallknecht et al. 1985).
In Central and South America and in Mexico, serotypes NJ and Indiana 1 have been associated with VS in cattle, horses, and swine annually. Subtypes Indiana 2 and Indiana 3 were isolated from mites from rice rats in Trinidad (Jonkers et al. 1964) and in outbreaks of VS in Argentina and Brazil (Garcia Perazzi et al. 1963), respectively. Indiana 2 and Indiana 3 (Federer et al. 1967) occur in Central and South America, respectively, but have not been reported to produce clinical disease in swine under field conditions. There are numerous other serotypes of vesiculoviruses (House and Wilks 1983; Tesh et al. 1983; Travassos da Rosa et al. 1984) but none has been reported to cause vesicular disease in swine under natural conditions.
CLINICAL SIGNS
[edit]The clinical signs of FMD, VS, VES, SVD, and SMSV cannot be distinguished from each other in the field. Generally, within 1–5 days postexposure, the body temperature rises sharply to 40.5˚C or higher. Areas of the epithelium become blanched, followed by the formation of vesicles and erosions after loss of the epithelium. Vesicles up to 3 cm in diameter may be found on the snout, with vesicular lesions extending into the nares, on the lips, tongue, hard and soft palate, coronary band, on the soft tissues of the feet (interdigital clefts and bulbs), and soft tissues around the dewclaws (see Figs. 25.1–25.4). Vesicular lesions may occur in other areas of the skin, especially where there is mechanical pressure or abrasion. Nursing sows may have vesicles on the teats. Slobbering and chomping are common. Pregnant sows may abort due to fever. The viruses of FMD, SVD, VES, and VS are not reported to infect the fetus.




Vesicles yield a serous fluid if they rupture (usually 6–24 hours after formation), and the epithelial layers above the stratum germinativum may detach. Eroded, hyperemic, hemorrhagic lesions are present. The hoof may become detached if the vesicles have coalesced around the coronary band. Severe laminitis may be followed by a chronically deformed foot. After the initial stage of infection, the animal’s temperature may drop to 40˚C. The lesions heal within 7–14 days if no secondary bacterial infection occurs.
FMD infects many or all of the exposed animals with no immunity in temperate climates or confined quarters. In partially immune populations or in tropical climates, morbidity may be decreased. VES is moderately contagious, with highly pathogenic strains affecting greater numbers of animals. With SVD, the entire herd may not be affected, and subclinical cases may occur. VS may spread horizontally in swine in confined quarters.
Low mortality (<5%) is usually seen with vesicular diseases. In young animals infected with FMDV or SVDV, myocardial necrosis may occur and mortality may reach 50–100%. There have been occasional reports of specific FMD isolates causing high mortality in mature animals. Cattle affected with a strain of C4 in Argentina, sheep in northern Africa affected with a strain of O1, and mountain gazelles in Israel affected with another strain of O1 (Shimshony et al. 1986) represent some examples of 50% or greater mortality in adult animals.
PATHOGENESIS
[edit]Foot-and-Mouth Disease
[edit]The major route of transmission for FMDV is aerosol (Sellers and Parker 1969; Donaldson et al. 1970; Thomson 1995). Relatively large aerosol particles (3–6 microns or greater) adhere to the upper respiratory tract, while smaller particles (3 microns or less) reach the lower respiratory tract. Viral nucleic acid, as detected by in situ hybridization, was widespread in epithelial tissues of cattle only 6 hours after aerosol exposures (Brown et al. 1992). In swine following intradermal inoculation in the snout, widespread FMDV nucleic acid was detected by in situ hybridization on the first sampling 24 hours after infection (Brown et al. 1995). Langerhans cells may be the primary means of this rapid distribution of virus. The pattern of distribution is "segmentally diffuse." Following seeding of secondary targets (epithelium, mucosa, and myocardium), the virus replicates, and viremia generally persists for 3-5 days (Sutmoller and McVicar 1976). Within 2-3 days, vesicles develop where stress and mechanical abrasions occur. In swine this includes the snout, feet, dewclaws, skin over the joints and pressure points of the extremities, mouth, tongue, and teats of nursing sows.
In addition, FMDV replication in the epithelial cells of the mammary gland is well documented. The FMDV is generally shed in the milk of cattle for up to 10 days postexposure, corresponding to the development of virus-neutralizing antibodies (Blackwell et al. 1982, 1983). In some cattle, however, the virus may be shed in the milk for up to 7 weeks, even in the presence of neutralizing antibodies in the serum (Burrows et al. 1971). Similar patterns of virus shedding in milk probably occur in swine. The virus is found in large quantities in oral and respiratory secretions 2-7 days postexposure. In young animals severe myocardial necrosis may occur.
Swine Vesicular Disease
[edit]Lesions are evident 3-11 days after eating contaminated food (McKercher and Graves 1981) and as early as 2-4 days after experimental inoculation (Burrows et al. 1973; Lai et al. 1979). The first lesions generally appear on the coronary band. A fever, which persists for up to 5 days, essentially coincides with the development of a cell-free viremia and vesicular lesions. The peak viremia occurs 2-4 days postexposure and persists for about 7 days. The virus persists for at least 7 days in the tissues of the snout, tongue, coronary band, tonsil, cardiac muscle, and central nervous system. The stratum spinosum is the primary site of viral replication in the epithelium. A nonsuppurative meningoencephalitis most frequently occurs in the cerebrum, thalamus, brain stem, and olfactory lobes. Necrosis and an inflammatory reaction occur in the endocardium and myocardium (Lai et al. 1979).
Vesicular Exanthema of Swine
[edit]VES is primarily introduced by feeding contaminated raw garbage. However, oral infection with VESV is estimated to require 100-1000 times the amount of virus needed to produce a lesion by intradermal inoculation into the snout (Mott et al. 1953). Following entry into the epithelium via an abrasion, the virus multiplies in the stratum germinativum of the epidermis. Hydropic degeneration occurs, followed by necrosis of infected cells. Intracellular edema (hydropic degeneration) and intercellular edema account for vesicle formation (Madin and Traum 1955). Virus spreads from cell to cell as the infection progresses. Local lymph nodes may become involved, with extensive lymphocyte destruction and congestion; a low-level viremia occurs and may account for some secondary lesions.
Vesicular Stomatitis
[edit]VSV apparently requires introduction into the epithelium through insect bites or via fomites into minor abrasions of the oral or nasal mucosa, teats, or feet (Patterson et al. 1955). Virus replicates in the stratum germinativum. A vesicle and an exudate form, usually 2-3 days postinoculation. Without secondary infection, which may involve the subcutaneous tissue, the lesion heals in 1-2 weeks.
The presence of a viremia is controversial in VSV infection. Early literature indicates that there may be a viremia. However, this has not been confirmed by more recent reports. In pigs, virus was isolated from local lymph nodes but not blood following intradermal inoculation in the snout to approximate the natural route of infection (Redelman et al. 1989).
LESIONS
[edit]The principal lesion, the vesicle, of FMD, SVD, VES, and VS is identical by gross pathology (Jubb et al. 1985). Vesicles occur in the epithelium of the oral and stratified squamous nasal mucosa, feet, teats, pressure points on the extremities, interdigital spaces, eyelids, and around the coronary bands.
A vesicular lesion begins as a small blanched area and progresses to a blanched, slightly raised area that expands as vesiculation progresses. The epithelium may separate from the stratum basale, leaving a red lesion with shreds of torn epithelium. Vesicular fluid may leak through the stratum corneum without the formation of a true vesicle (Seibold and Sharp 1960). Since vesicles are most often seen at the site of mechanical stress, they usually rupture quickly, leaving a red erosion. Often, lesions are contaminated with dirt and debris (Fig. 25.2), resulting in infection with opportunistic bacteria. The foot lesions, especially of VES, may be associated with a cellulitis with persistent swelling, resulting in lameness and local lymph node involvement. In young animals infected with FMDV, myocardial necrosis may produce pale necrotic areas referred to as "tiger heart."
Histopathology
[edit]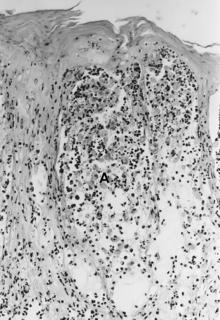
The histopathology of FMD, SVD, and VES is considered quite similar. The lesions begin in the stratum spinosum, where vesicles form because of hydropic degeneration and intercellular edema. Keratinocytes die, become spherical, and float as single or clustered cells in the vesicular fluid (spongiosa) (Fig. 25.5A). The stratum basale essentially remains intact but may be somewhat disrupted by VESV infection (Mebus 1977).
The histopathology of VS in cattle has been carefully studied by Seibold and Sharp (1960) and described by Jubb et al. (1985) and is probably equivalent for swine. The lesions develop in the stratum spinosum with intercellular edema that stretches the intercellular bridges (desmosomes). Cells remain attached at the ends parallel to the stratum basale but are separated lengthwise by intercellular edema, giving a reticular or “Japanese lantern” appearance (Fig. 25.5B). Cells become necrotic in the later stages of the lesion. The epithelial layers above the stratum basale separate in about 30% of the cases. Loss of vesicular fluid through the stratum corneum occurs frequently, and vesicles may not be observed.
DIAGNOSIS
[edit]The diagnosis of FMD, SVD, VES, and VS must be conducted in the laboratory, for the diseases are clinically indistinguishable from each other. In countries where vesicular diseases are endemic, national animal disease diagnostic laboratories are generally available. Samples may be shipped to the World Reference Laboratory for Foot-and-Mouth Disease at Pirbright, Great Britain, or to regional vesicular disease diagnostic laboratories including the Foreign Animal Disease Diagnostic Laboratory (FADDL) at the PIADC in Plum Island, New York, United States, or the Pan American Foot-and-Mouth Disease Laboratory in Rio de Janeiro, Brazil. The director of the laboratory should be contacted before shipment. Permits may be needed for shipment to some laboratories, and ever-changing international shipping regulations for diagnostic samples must be followed.
Samples from acutely ill swine should include vesicular tissue and, if possible, vesicular fluid from gently washed, active lesions on the tongue, lips, gums, nares, feet, dewclaws, extremities, or teats. Heparinized and clotted blood should be collected for virus isolation and serology, respectively. Clotted blood for serum should be collected from recovered animals. Necropsy samples of tissues for virus isolation should include vesicular lesions and, particularly from newborn or young animals, skeletal muscle and myocardium. These samples should be shipped on wet ice if they will arrive at a diagnostic laboratory within 24 hours. If a longer shipping time is anticipated, freezing the tissue in airtight vials and shipping on dry ice is recommended. For histopathology, a complete set of tissues representing all organ systems, but with emphasis on organs with lesions, should be collected and preserved in buffered 10% formalin.
In swine, the differential diagnosis of FMD, SVD, VES, and VS should include swine pox, hog cholera, pseudorabies, porcine parvovirus infection (Kresse et al. 1985), parsnip or celery contact dermatitis (Montgomery et al. 1987), chemical burns, trauma, and mycotic stomatitis.
Epithelial and vesicular fluid samples are tested by complement fixation (CF) or an antigen capture ELISA for the presence of viral antigens of FMD, SVD, and VS. ELISA can detect and identify serotypes of FMD viral antigens at lower concentrations than the CF test under experimental (Roeder and LeBlanc Smith 1987) and field conditions (Westbury et al. 1988). The results of these tests can be available within 4 hours of receipt of the sample. Virus isolation in cell cultures (Snowdon 1966; House and House 1989) of suckling mice, electron microscopy (EM), and animal host inoculation studies may be done. In nonenzootic areas, virus isolation should be performed. A positive FMDV isolation with serotype identification may be completed as early as 24-72 hours after receipt of the sample. A negative virus isolation study may take up to 2 weeks, as multiple passages may be required.
Serum samples may be tested for precipitating antibodies to the FMD VIAA, for neutralizing antibodies to FMDVs, SVDV, VESVs, and VSVs, and for CF antibodies to VSVs, with results available in 1-3 days. Virus-neutralizing antibodies are serotype-specific for FMDV, and animals infected for the first time characteristically develop the highest titer against the homologous subtype. Antibodies to FMD VIAA were used historically to differentiate the immune response to active infection from that of vaccination. However, repeated vaccination with killed FMD vaccine may elicit a transient antibody response to VIAA (Pinto and Garland 1979). An ELISA employing baculovirus-expressed FMD nonstructural proteins 3D and 2C can be used to differentiate vaccinated from convalescent animals (Meyer et al. 1997).
RNA fingerprinting, which uses ribonuclease T1 to specifically cleave the RNA at unique sites and produce a spectrum of different oligonucleotides, has been used to study the relationship of FMDVs (Frisby et al. 1976). However, nucleotide sequencing, which provides the order of the nucleotides in the native RNA, is a more precise tool to study the variable areas of the genome of FMDV (Beck and Strohmaier 1987; Westbury et al. 1988; Marquardt and Adam 1990). The polymerase chain reaction (Meyer et al. 1991; Donn et al. 1994; Marquardt et al. 1995), combined with nucleotide sequencing, provides definitive epidemiological information on the origin of viral strains.
TREATMENT
[edit]No specific treatments are known for the vesicular diseases. Palliative measures include feeding soft feeds, removing animals from hard-surfaced or concrete floors, and providing access to clean water. Providing hygienic conditions, using antiseptic solutions on affected epithelium, and administering antibiotics may help prevent and control secondary infections.
PREVENTION
[edit]Exotic Vesicular Diseases
[edit]It is imperative to prevent the infection of swine with FMDV since they can produce tremendous infectious aerosols. Prevention of FMD and SVD is accomplished by strictly regulating movement of animals and animal products. Swine from FMD-endemic areas were first imported into the United States from China in 1989 using the methods developed for cattle importation (House et al. 1983). Swine semen has also been imported using testing and quarantine procedures. The importation of swine embryos may be complicated by the depth of sperm tracts and other undefined characteristics of porcine embryos that may cause adherence of microbial agents to the zona (Callis 1996).
Regulations for the international movement of animal products other than germ plasm are based on virus inactivation studies. Inactivation of FMDV and SVDV in meat products has been reviewed (Blackwell 1984). Pork products from swine infected with FMDV and SVDV (McKercher et al. 1985, 1987) and from swine infected with FMDV, SVDV, African swine fever virus, and hog cholera virus (classic swine fever virus) (Mebus et al. 1993a, b) were free of virus when subjected to prolonged curing processes. Virus in the milk of FMDV-infected cattle was inactivated by pasteurization at ultrahigh temperatures for a short time (148°C for 2 seconds) (Cunliffe et al. 1979).
Regulations for cooking of garbage fed to swine resulted in the control and eradication of VES (Madin and Traum 1953). Swine fed seal meat or other marine-origin feed supplements could become infected with caliciviruses, but such practices are no longer in use and VES has not recurred.
Domestic Vesicular Diseases
[edit]Vaccination has not been used to prevent VS in swine in the United States (Buisch 1983). The segregation of swine from infected cattle, horses, and wildlife, including feral swine, in epizootic regions is a practical suggestion. The role of insects in the spread of VS justifies an insect control program during an epizootic.
CONTROL
[edit]In case of an outbreak of exotic vesicular disease in swine, the USDA emergency disease guidelines call for stamping out of the disease upon identification of the exotic agent. The plan calls for immediate quarantine of the affected premises, restriction of animal movement, public-alert statements, eradication by slaughter, and close communication and cooperation with industry.
Today, the loss of genetic material and animal protein, environmental regulations, and animal welfare concerns warrant the consideration of vaccination as a tool for controlling and eradicating FMD.
The North American Foot-and-Mouth Disease Vaccine Bank, maintained by USDA in cooperation with Canada and Mexico and other international FMD vaccine banks, has reserves of inactivated, safety- and potency- tested FMD vaccine antigens available to be formulated into FMD vaccines. Vaccinated animals could be permanently identified and their movement restricted. Vaccines for FMD, used throughout the world in FMD-endemic areas, are inactivated, adjuvanted vaccines. These vaccines are produced in BHK (baby hamster kidney) suspension cell cultures and inactivated with ethylenamine derivatives. Vaccination with the prevalent serotypes 2-4 times a year is recommended. The traditional aluminum hydroxide-saponin adjuvanted FMD vaccines used for cattle are less efficacious in swine than are oil-adjuvanted FMD vaccines (McKercher and Giordano 1967).
No genetically engineered vaccines for FMD are commercially available, and their practicality is uncertain. Experimentally, genetic sequences for the key immunogenic FMD VP1 were cloned into Escherichia coli, and VP1 was successfully expressed (Kleid et al. 1981). The cloned protein protected vaccinated cattle and swine against a virulent challenge inoculation. The system was successful with selected subtypes of serotypes A and C, but for serotype O, the cloned proteins did not provide consistent protection. The amino acid sequence of critical polypeptides, which could induce neutralizing antibodies to serotype O, was deduced from the nucleotide sequence. Synthetic polypeptides protected guinea pigs (Bittle et al. 1982) and cattle (DiMarchi et al. 1986) against a virulent challenge inoculation. Roosien et al. (1990) have synthesized empty capsids of FMDV in insect cells using baculovirus-expression vectors, but there are no reports of their efficacy.
Inactivated VS vaccines were produced and provisionally licensed for use in cattle during the 1982-83 epizootic, and for horses and cattle in 1995. Efficacy data on these products are not available. There is no vaccine available for SVD, since eradication is the preferred method of control.
REFERENCES
[edit]Acharya, R.; Fry, E.; Stuart, D.; Fox, D; Rowlands, D.; and Brown, F. 1989. The three-dimensional structure of footand- mouth disease virus at 2.9A revolution. Nature 337:709-716.
Bachrach, H. L. 1977. Foot-and-mouth disease virus: Properties, molecular biology and immunogenicity. In Beltsville Symposium in Agricultural Research I. Virology in Agriculture. Ed. J. A. Romberger. Montclair, N.J.: Allanheld, Osmum & Co., pp. 46-49. Bankowski, R. A. 1965. Vesicular exanthema. Adv Vet Sci 10:23-64.
Bankowski, R. A.; Perkins, A. G.; Stuart, E. E.; and Kummer, M. 1955. Epizootiology of vesicular exanthema in California. In Proc 59th Annu Meet US Livestock Sanit Assoc, pp. 356-367. Barlough, J. E.; Berry, E. S.; Smith, A. W.; and Skilling, D. E. 1987. Prevalence and distribution of serum neutralizing antibodies to Tillamook (bovine) calicivirus in selected populations of marine mammals. J Wildl Dis 23:45-51.
Beck, E., and Strohmaier, K. 1987. Subtyping of European foot-and-mouth disease virus strains by nucleotide sequence determination. J Virol 61:1621-1629.
Berry, E. S.; Skilling, B. S.; Barlough, J. E.; Vedros, N. A.; Gage, L. J.; and Smith, A. W. 1990. New marine calicivirus serotype infective for swine. Am J Vet Res 51:1184-1187.
Bittle, J. L.; Houghten, R. A.; Alexander, H.; Shinnick, T. M.; Sutcliffe, J. G.; Leaner, R. A.; Rowlands, D. J.; and Brown, F. 1982. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide produced from viral nucleotide sequence. Nature 298:30. Blackwell, J. H. 1984. Foreign animal disease agent survival in animal products: Recent developments. J Am Vet Med Assoc 184:674-679.
Blackwell, J. H.; McKercher, P. D; Kosikowski, F. V; Carmichael, L. E.; and Gorewit, R. C. 1982. Concentration of foot-and-mouth disease virus in milk of cows infected under simulated field conditions. J Dairy Sci 65:1624-1631.
___. 1983. Histological and histochemical characterization of mammary gland tissue of cows infected with foot-and-mouth disease by contact exposure. Res Vet Sci 35:106-113.
Brooksby, J. B. 1982. Portraits of viruses: Foot-and-mouth disease virus. Intervirology 18:1-23.
Brown, C. C.; Meyer, R. F.; Olander, H. J.; House, C.; and Mebus, C. A. 1992. A pathogenesis study of foot-andmouth disease in cattle, using in situ hybridization. Can J Vet Res 56:189-193.
Brown, C. C.; Olander, H. J.; and Meyer, R. F. 1995. Pathogenesis of foot-and-mouth disease in swine, studied by in-situ hybridization. J Comp Path 113:51-58.
Brown, F.; Goodridge, D.; and Burrows, R. 1976. Infection of man with swine vesicular disease virus. J Comp Path 86:409-414.
Buisch, W. W. 1983. Fiscal year 1982-1983 vesicular stomatitis outbreak. In Proc 87th Annu Meet US Anim Health Assoc, pp. 78-84. Bulloch, W. 1927. De contagione et contagiosis morbis et curatione. J Comp Path 40:75-76.
Burrows, R.; Mann, J. A.; Greig, A.; Chapman, W. G.; and Goodridge, D. 1971. The growth and persistence of footand- mouth disease virus in the bovine mammary gland. J Hyg (Camb) 69:307-321.
Burrows, R; Greig, A.; and Goodridge, D. 1973. Swine vesicular disease. Res Vet Sci 15:141-144.
Callis, J. J. 1996. Evaluation of the presence and risk of footand- mouth disease virus by commodity in international trade. Res Sci Tech Off Int Epiz 15:1075-1085.
Chu, R. M.; Moore, D. M.; and Conroy, J. D. 1979. Experimental swine vesicular disease, pathology and immunofluorescence studies. Can J Comp Med 43:29-30.
Comer, J. A.; Tesh, R. B.; Modi, G. B.; Coin, J. T.; and Nettles, V. F. 1990. Vesicular stomatitis virus New Jersey serotype: Replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am J Trop Med Hyg 42:483-490.
Coombs, G. P. 1973. A study of swine vesicular disease in England. In Proc 77th Annu Meet US Anim Health Assoc, pp. 332-335. Cotton, W. E. 1927. Vesicular stomatitis. Vet Med 22:169-175.
Cottral, G. E.; Gailiunas, P.; and Cox, B. F. 1968. Foot-andmouth disease virus in semen of bulls and its transmission by artificial insemination. Arch Gesamte Virusforsch 23:362-377.
Cunliffe, H. R.; Blackwell, J. H.; Dors, R.; and Walker, J. S. 1979. Inactivation of milk-borne foot-and-mouth disease virus at ultrahigh temperatures. J Food Prot 42(2):135-137. Cupp, E. W.; Mare, J.; Cupp, M. S.; and Ramberg, F. B. 1992. Biological transmission of vesicular stomatitis virus (New Jersey) by Simulium vittatum (Diptera: Simulidae) J Med Ent 29:137-140.
Dawe, P. S.; Sorenson, K.; Ferris, N. P.; Barnett, I. T. R.; Armstong, R. M.; and Knowles, N. J. 1994. Transmission of foot-and-mouth disease from carrier African buffalo (Syncerus caffer) to cattle under experimental conditions in Zimbabwe. Vet Rec 134:211-215.
DiMarchi, R.; Brooke, G.; Gale, C.; Cracknell, V.; Dale, T.; and Mowat, N. 1986. Protection of cattle against footand- mouth disease by a synthetic peptide. Science 232:639-641.
Domingo, E.; Diez, J.; Martinez, M. A.; Hernandez, J.; Holguin, A.; Borrego, B.; and Mateu, M. G. 1993. New observations on antigenic diversification of RNA viruses: Antigenic variation is not dependent on immune selection. J Gen Virol 74:2039-2045.
Donaldson, A. I., and Ferris, N. P. 1980. Sites of release of airborne foot-and-mouth disease virus from infected pigs. Res Vet Sci 29(3):315-319. Donaldson, A. I.; Herniman, K. A. J.; Parker, J.; and Sellers, R. F. 1970. Further investigations on the airborne excretion of foot-and-mouth disease virus. J Hyg (Camb) 68:557-564.
Donaldson, A. I.; Gibson, C. F.; Oliver, R.; Hamblin, C.; and Kitching, R. P. 1987. Infection of cattle by airborne footand- mouth disease virus: Minimal doses with O1 and SAT 2 strains. Res Vet Sci 43:339-346.
Donn, A.; Martin, L. A.; and Donaldson, A. I. 1994. Improved detection of persistent foot-and-mouth disease infection in cattle by the polymerase chain reaction. J Virol Meth 49:179-186.
Dunn, C. S., and Donaldson, A. I. 1997. Natural adaption to pigs of a Taiwanese isolate of foot-and-mouth disease virus. Vet Rec 141:174-175.
Federer, K. E.; Burrows, R.; and Brooksby, J. B. 1967. Vesicular stomatitis virus: The relationship between some strains of the Indiana serotypes. Res Vet Sci 8:103-117.
Fox, G.; Parry, N. R.; Barnett, P. V.; McGinn, B.; Rowlands, D. J.; and Brown, F. 1989. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (argenine-glycine-aspartic acid). J Gen Virol 70:625-637.
Francki, R. B.; Fauquet, C. M.; Knudsen, D. L.; and Brown, F. 1991a. Classification and Nomenclature of Viruses. Fifth Report of the International Committee on Taxonomy of Virus. Arch Virol, Suppl 2. New York: Springer Verlag, pp. 250-254.
___. 1991b. Classification and Nomenclature of Viruses. Fifth Report of the International Committee on Taxonomy of Virus. Arch Virol, Suppl 2. New York: Springer Verlag, pp. 300-302.
___. 1991c. Classification and Nomenclature of Viruses. Fifth Report of the International Committee on Taxonomy of Virus. Arch Virol, Suppl 2. New York: Springer Verlag, pp. 320-326. Frisby, D. P.; Newton, C.; Carey, N. H.; Fellner, P.; Newman, J. F. E.; Harris, T. J. R.; and Brown, F. 1976. Oligonucleotide mapping of picornavirus RNAs by two-dimensional electrophoresis. Virol 71:379-388.
Garcia Perazzi, A. J.; Caggiano, C. H.; and Fernandez, A. A. 1963. Publication Tecnica No 2. CADEFA, Ministry of Agriculture, Buenos Aires, Argentina. Gebauer, F.; De La Torro, J. C.; Gomes, I.; Mateu, M. G.; Barahona, H.; Tiraboschi, B.; Bergmann, I.; Demello, P.; and Domingo, E. 1988. Rapid selection of genetic and antigenic variants of foot-and-mouth disease virus during persistence in cattle. J Virol 62:2041-2049.
Graves, J. H. 1973. Serological relationship of swine vesicular disease virus and Coxsackie B-5 virus. Nature 245:314. Hedger, R. S., and Condy, J. B. 1985. Transmission of foot and mouth disease from African buffalo virus carriers to bovines. Vet Rec 117:205. Herniman, K. A. J.; Medhurst, P. M.; Wilson, J. N.; and Sellers, R. F. 1973. The action of heat, chemicals and disinfectants on swine vesicular disease virus. Vet Rec 93:620-624.
House, C., and House, J. A. 1989. Evaluation of techniques to demonstrate foot-and-mouth disease virus in bovine tongue epithelium: Comparison of the sensitivity of cattle, mice, primary cell cultures, cryopreserved cell cultures, and established cell lines. Vet Microbiol 20:99-109.
House, J. A., and Wilks, C. R. 1983. Studies on exotic vesiculoviruses. In Proc 87th Ann Meet US Animal Health Assoc, pp. 276-288. House, J. A.; Yedloutschnig, R. J.; Dardiri, A. H.; Herrick, D. E.; and Acree, J. A. 1983. Procedures used for the importation of Brazilian Zebu cattle into the United States. Proc 26th Annu Meet Am Assoc Vet Lab Diagn, pp. 13-24. Jonkers, A. H.; Shope, R. E.; Aitken, T. H. G.; and Spence, L. 1964. Cocal virus, a new agent in Trinidad related to vesicular stomatitis virus. Am J Vet Res 25:236-242.
Jubb, K. V. F.; Kennedy, P. C.; and Palmer, N. 1985. Pathology of Domestic Animals, vol. 2. New York: Academic Press, pp. 90-110. Kleid, D. G.; Yansura, D.; Small, B.; Dowbenko, D.; Moore, D. M.; Grubman, M. J.; McKercher, P. D.; Morgan, D. O.; Robertson, B. H.; and Bachrach, H. L. 1981. Cloned viral protein vaccine for foot-and-mouth disease: Responses in cattle and swine. Science 214:1125-1129.
Kresse, J. I.; Taylor, W. D.; Stewart, W. C.; and Eernissee, K. A. 1985. Parvovirus infection in pigs with necrotic and vesicle-like lesions. Vet Microbiol 10:525-531.
Lai, S. S; McKercher, P. D.; Moore, D. M.; and Gillespie, J. H. 1979. Pathogenesis of swine vesicular disease in pigs. Am J Vet Res 40:463-468.
Loeffler, F., and Frosch, P. 1898. Zentralbl Bakt 1 Orig 28:371. Lubroth, J.; Grubman, M. J.; Burrage, T. G.; Newman, J. F. E.; and Brown, F. 1996. Absence of protein 2C from clarified foot-and-mouth disease virus vaccine provides the basis for distinguishing convalescent from vaccinated animals. Vaccine 14:419-427.
Madin, S. H., and Traum, J. 1953. Experimental studies with vesicular exanthema of swine. II. Studies on stability. Vet Med 48:443-450.
___. 1955. Vesicular exanthema of swine. Bacteriol Rev 19:6-19.
Mann, J. A., and Hutchings, G. H. 1980. Swine vesicular disease: Pathways of infections. J Hyg 84:355-363.
Mann, J. A.; Burrows, R.; and Goodridge, D. 1975. Mild subclinical infections with swine vesicular disease virus. Bull Off Int Epiz 83:117-122.
Maragon, S.; Facchin, F.; Moutou, I.; Massirio, G.; Vincenzi, G.; and Davies, G. 1994. The 1993 Italian foot-andmouth disease epidemic: Epidemiological features of the four outbreaks identified in Verona province (Ventro region). Vet Rec 135:53-57.
Marquardt, O., and Adam, K. 1990. Foot-and-mouth disease virus subtyping by sequencing VP1 genes. Vet Microbiol 23:175-183.
Marquardt, O.; Straub, O. C.; Ahl, R.; and Haas, B. 1995. Detection of foot-and-mouth disease virus in nasal swabs of asymptomatic cattle by RT-PCR within 24 hours. J Virol Meth 53:255-261.
McKercher, P. D., and Giordano, A. R. 1967. Foot-andmouth disease in swine. I. The immune response of swine to chemically treated and nontreated foot-andmouth disease virus. Arch Gesamte Virusforsch 20:39-53.
McKercher, P. D., and Graves, J. H. 1981. Swine vesicular disease. In CRC Handbook Series in Zoonoses, Sec B. Viral Zoonoses, vol. 2. Ed. J. H. Steele. Boca Raton, Fla.: CRC Press, pp. 161-167. McKercher, P. D.; Blackwell, J. H.; Murphy, R.; Callis, J. J.; Panina, G. F.; Civardi, A.; Bugnetti, M.; Desimone, F.; and Scatozza, F. 1985. Survival of swine vesicular disease virus in "Prosciutto di Parma" (Parma Ham). Can Inst Food Sci Technol J 18:163-167.
McKercher, P. D.; Yedloutschnig, R. J.; Callis, J. J.; Murphy, R.; Panina, G. F.; Civardi, A.; Bugnetti, M.; Foni, E.; Laddamada, A.; Scarano, C.; and Scatozza, F. 1987. Survival of viruses in "Prosciutto di Parma" (Parma Ham). Can Inst Food Sci Technol J 20:267-272.
McVicar, J. W.; Eisner, R. J.; Johnson, L. A.; and Pursel, V. G. 1978. Foot-and-mouth disease and swine vesicular disease viruses in boar semen. In Proc 81st Annu Meet US Anim Health Assoc, pp. 221-230. Mebus, C. A. 1977. Ulcerative diseases of animals with an infectious etiology. J Oral Path 7:365-371.
Mebus, C. A.; House, C.; Ruiz Gonzalvo, F.; Pineda, J. M.; Tapiador, J.; Pire, J. J.; Bergada, J.; Yedloutschnig, R. J.; Sahu, S.; Becerra, V.; and Sanchez-Vizcaino, J. M. 1993a. Survival of foot-and-mouth disease, African swine fever and hog cholera viruses in Spanish Serrano cured hams and Iberian cured hams, shoulders and loins. Food Microbiol 10:133-143.
Mebus, C. A.; House, C.; Ruiz Gonzalvo, F.; Pineda, J. M.; Tapiador, J.; Pire, J. J.; Bergada, J.; Yedloutschnig, R. J.; and Sanchez-Vizcaino, J. M. 1993b. Survival of swine vesicular disease virus in Spanish Serrano cured hams and Iberian cured hams, shoulders and loins. Food Microbiol 10:263-268.
Meyer, R. F.; Brown, C. C.; House, C.; House, J.; and Molitor T. W. 1991. Rapid and sensitive detection of foot-andmouth disease virus in tissues by enzymatic RNA amplification of the polymerase gene. J Virol Methods 34:161-172.
Meyer, R. F.; Babcock, G. D.; Newman, J. F. E.; Burrage, T. G.; Toohey, K.; Lubroth, J.; and Brown, F. 1997. Baculovirus expressed 2C of foot-and-mouth disease virus has the potential for differentiating convalescent from vaccinated animals. J Virol Meth, in press. Montgomery, J. F.; Oliver, R. E.; and Poole, W. S. H. 1987. A vesiculo-bullous disease in pigs resembling foot-andmouth disease. I. Field cases. NZ Vet J 35:21-26.
Mott, L. O.; Patterson, W. C.; Songer, J. R.; and Hopkins, S. R. 1953. Experimental infections with vesicular exanthema. II. Feeding of viral suspensions and infected tissues. In Proc 57th Annu Meet US Livest Sanit Assoc, pp. 349-360. Mulhern, F. J. 1953. Present status of vesicular exanthema eradication program. In Proc 57th Annu Meet US Livest Sanit Assoc, pp. 326-333. Nardelli, L.; Lodetti, E.; Gualandi, G. L.; Burrows, R.; Goodridge, D.; Brown, F.; and Cartwright, B. 1968. A foot-and-mouth disease syndrome in pigs caused by an enterovirus. Nature 219:1275-1276.
Newman, J. F. E.; Piatti, P. G.; Gorman, B. M.; Burrage, T. G.; Ryan, M. D.; Flint, M.; and Brown, F. 1994. Foot-andmouth disease virus particles contain replicase protein 3D. Proc Natl Acad Sci USA 91:733-737.
Patterson, W. C.; Jenny, E. W; and Holbrook, A. A. 1955. Experimental infections with vesicular stomatitis in swine. I. Transmission by direct contact and feeding infected meat scraps. In Proc 59th Annu Meet US Livest Sanit Assoc, pp. 368-378. Pereira, H. G. 1977. Subtyping of foot-and-mouth disease virus. In Developments in Biological Standardization. Ed. C. Mackowiak and R. H. Regamey. Basel: S. Karger, vol. 35, pp. 167-174. Pinto, A. A., and Garland, A. J. M. 1979. Immune response to virus-infection-associated (VIA) antigen in cattle repeatedly vaccinated with foot-and-mouth disease virus inactivated by formalin or acetylethyleneimine. J Hyg (Camb) 82:41-50.
Redelman, D.; Nichol, S.; Kleeforth, R.; Van der Matten, M.; and Whetstone, C. 1989. Experimental vesicular stomatitis virus infection of swine: Extent of infection and immunological response. Vet Immunol Immunopathol 20:345-361.
Roeder, P. L., and Leblanc Smith, P. M. 1987. Detection and typing of foot-and-mouth disease virus by enzymelinked immunosorbent assay: A sensitive, rapid, and reliable technique for primary diagnosis. Res Vet Sci 43:225-232.
Roosien, J.; Belsham, G. J.; Ryan, M. D.; King, A. M. Q.; and Vlak, J. M. 1990. Synthesis of foot-and-mouth disease virus capsid proteins in insect cells using baculovirus expression vectors. J Gen Virol 71:1703-1711.
Sawyer, J. C.; Madin, S. H.; and Skilling, D. E. 1978. Isolation of San Miguel sea lion virus from samples of an animal food product produced from Northern fur seal (Callorhinus ursinus) carcasses. Am J Vet Res 39:137-139.
Schoening, H. W. 1943. Vesicular stomatitis in swine. In Proc 47th Annu Meet US Livest Sanit Assoc, pp. 85-86. Seibold, H. R., and Sharp, J. B. 1960. A revised concept of the pathological changes of the tongue in cattle with vesicular stomatitis. Am J Vet Res 21:35-51.
Sellers, R. F., and Herniman, K. A. J. 1974. The airborne excretion by pigs of swine vesicular disease virus. J Hyg (Camb) 72:61-65.
Sellers, R. F., and Parker, J. 1969. Airborne excretion of footand- mouth disease virus. J Hyg (Camb) 67:671-677.
Sellers, R. F.; Herniman, K. A. J.; and Donaldson, A. I. 1971. The effects of killing or removal of animals affected with foot-and-mouth disease on the amounts of airborne virus present in loose boxes. Brit Vet J 127:358-365.
Shimshony, A.; Orgad, U.; Baharv, D.; Prudovsky, S.; Yakobson, B.; Bar Moshe, B.; and Dagan, D. 1986. Malignant foot-and-mouth disease in mountain gazelles. Vet Rec 119:175-176.
Smith, A. W., and Latham, A. B. 1978. Prevalence of vesicular exanthema of swine antibodies among feral mammals associated with the southern California coastal zones. Am J Vet Res 39:291-296.
Smith, A. W.; Akers, T. G.; Madin, S. H.; and Vedros, N. A. 1973. San Miguel sea lion virus isolation, preliminary characterization, and relationships to vesicular exanthema of swine virus. Nature 244:108-109.
Smith, A. W.; Prato, C. M.; and Skilling, D. E. 1977. Characterization of two new serotypes of San Miguel sea lion virus. Intervirology 8:30-36.
Smith, A. W.; Akers, T. B.; Latham, A. B; Skilling, D. E.; and Bray, H. L. 1979. A new calicivirus isolated from a marine mammal. Arch Virol 61:255-259.
Smith, A. W.; Skilling, D. E.; Dardiri, A. H.; and Latham, A. B. 1980. Calicivirus pathogenic for swine: A new serotype isolated from opaleye Girella nigricans, an ocean fish. Science 209:940-941.
Smith, A. W.; Mattson, D. E.; Shilling, D. E.; and Schmitz, J. A. 1983a. Isolation and partial characterization of a calicivirus from calves. Am J Vet Res 44:851-855.
Smith, A. W.; Ritter, D. G.; Ray, G. C; Skilling, D. E.; and Wartzok, D. 1983b. New calicivirus isolates from feces of walrus (Odobenus rosmarus). J Wildl Dis 19:86-183.
Snowdon, W. A. 1966. Growth of foot-and-mouth disease virus in monolayer cultures of calf thyroid cells. Nature 210:1079-1080.
Stallknecht, D. E.; Nettles, V. F.; Fletcher, W. D.; and Erickson, G. A. 1985. Enzootic vesicular stomatitis New Jersey type in an insular feral swine population. Am J Epidemiol 122:876-883.
Sutmoller, P., and McVicar, J. W. 1976. Pathogenesis of footand- mouth disease: The lung as an additional portal of entry of the virus. J Hyg (Camb) 77:235-243.
Tesh, R. B.; Chaniotis, B. H.; and Johnson, K. M. 1971. Vesicular stomatitis virus (Indiana serotype) transovarial transmission by phlebotomine sandflies. Science 175:1477-1499.
Tesh, R. B.; Travassos da Rosa, A. P. A.; and Travassos da Rosa, J. S. 1983. Antigenic relationship among rhabdoviruses infecting terrestrial vertebrates. J Gen Virol 64:169-176.
Thomson, G. R. 1994. Foot-and-mouth disease. In Infectious Diseases of Livestock with Special Reference to Southern Africa. Ed. J. A. W. Coetzer, G. R. Thomson, and R. C. Tustin. vol. 2. New York: Oxford University Press, pp. 825-852.
___. 1995. Overview of foot and mouth disease in southern Africa. Rev Sci Tech Off Int Epiz 14:503-520.
Traum, J. 1934. Vesicular exanthema of swine. In Proc 12th Int Vet Congr, New York, vol. 2, pp. 5-9.
Travassos da Rosa, A. P. A.; Tesh, P. B.; Travassos da Rosa, J. S.; Herve, J. P.; and Main, A. J. 1984. Carajas and Maraba virus, two new vesiculoviruses isolated from phlebotomine sand-flies in Brazil. Am J Trop Med Hyg 33:999-1006.
Vosloo, W.; Bastos, A. D.; Kirkbride, E.; Esterhuysen, J. J.; Janse Van Rensburg, D.; Bengis, R. G.; Keet, D. W.; and Thomson, G. R. 1996. Persistent infection of African buffalo (Syncerus caffer) with SAT-type foot and mouth disease viruses: Rate of fixation of mutations, antigenic change and interspecies transmission. J Gen Virol 77:1457-1467.
Westbury, H. A.; Doughty, W. J.; Forman, A. J.; Suchinta Tangchaitrong; and Kongthan, A. 1988. A comparison of enzyme-linked immunosorbent assay, complement fixation, and virus isolation for foot-and-mouth disease diagnosis. Vet Microbiol 17:21-28.